Abstract
Conventional hemodialysis, which is based on the diffusive transport of solutes, is the most widely used renal replacement therapy. It effectively removes small solutes such as urea and corrects fluid, electrolyte and acid-base imbalance. However, solute diffusion coefficients decreased rapidly as molecular size increased. Because of this, middle and large molecules are not removed effectively and clinical problem such as dialysis amyloidosis might occur. Online hemodiafiltration which is combined by diffusive and convective therapies can overcome such problems by removing effectively middle and large solutes. Online hemodiafiltration is safe, very effective, economically affordable, improving session tolerance and may improve the mortality superior to high flux hemodialysis. However, there might be some potential limitations for setting up online hemodiafiltaration. In this article, we review the uremic toxins associated with dialysis, definition of hemodiafiltration, indication and prescription of hemodiafiltration and the limitations of setting up hemodiafiltration.
The most widely used renal replacement therapy is conventional hemodialysis based on diffusion. It has been recognized that uremic toxins have various molecular weight from small size to large one. Middle and large molecular uremic toxins have a limitation in solute removal by diffusional method only. The need for alternative therapies to better remove these solutes have become evident. Convective transport of solutes decreases slower than diffusive transport because sieving coefficients are less dependent on the molecular size. Combined convective and diffusive therapy, hemodiafiltration (HDF), is an ideal renal replacment therapy for replacing the natural kidney compared to other hemodialysis. However, there are some requirements to do HDF.
Impaired kidney cannot excrete a myriad of compounds and causes retention of uremic solutes related to deterioration of multiple biochemical and physiological functions1). The role of each solute has not been established in most cases. Uremic toxins can be subdivided by its size, solubility to water and protein bounded or not. A small sized molecule under 500 Daltons (Da) molecular weight and a large sized molecule is over 12,000 Da molecular weight. Middle molecules are between 500 and 12,000 Da molecular weight. For example, small water soluble compounds are urea (60 Da), creatinine (113 Da) and phosphate (134 Da). Middle molecules are parathyroid hormone (9,425 Da), β2-microglobulin (11,818 Da) and advanced glycosylation end products (2,000-6,000 Da). Protein-bound compounds are p-cresol, p-cresyl sulfate and indoles. Molecules are removed by diffusive, absorptive and convective transport. However, these middle and protein-bound uremic toxins cannot be removed sufficiently by only the diffusive method.
According to the "Consensus conference on Biocompatibility", hemodiafiltration is designed to remove accumulated metabolic products by a combination of diffusive and convective transport through a high-flux semi-permeable membrane. Fluid is removed by ultrafiltration and the volume of filtered fluid exceeding the desired weight loss is replaced by sterile, pyrogen-free infusion solution by online.
HDF is preferred to achieve better urea clearance, optimal weight, and improved phosphate levels. However, additional cost, vascular access and lack of machines, ultrapure water and trained staff are obstacles of HDF. The HDF machine and dialyzer, online ultrapure dialysis fluid, indication of HDF, mode and optimal dose, safety and cost-effectiveness are real practical problems.
Certified online HDF (Ol-HDF) machines are needed. The International Electrotechnical Commission (IEC) has published a standard (IEC 60601-2-16) for machines and compliance with this standard is required to obtain a CE (Communaut' Europeen) mark for equipment used to perform HDF2).
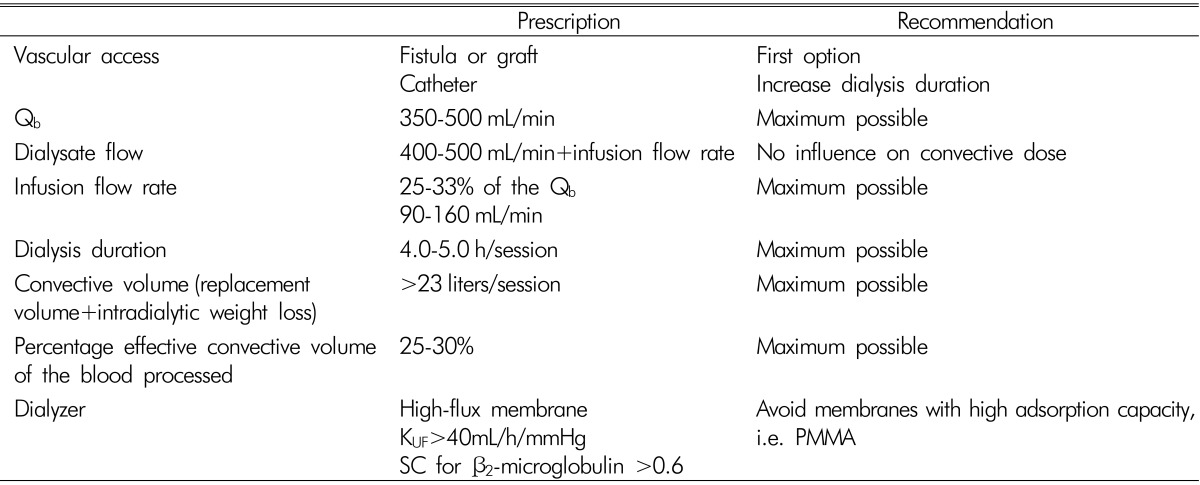
A high-flux dialyzer with an ultrafiltration coefficient higher than 20mL/mmHg/h/m2, sieving coefficient for β2-microglobulin greater than 0.6 and a percentage of effective convective transport greater than 20% of the total processed blood, is needed. The most adequate dialyzer refer to KUF≥40mL/h/mmHg, KoA urea >600 and β2-microglobulin >60mL/min with large surface area (1.50-2.10m2)3). However, individualized choice of dialyzer in HDF is needed because a high-flux dialyzer has diverse effects on removing middle molecules and the loss of essential proteins such as albumin.
The International Organization for Standardization (ISO) has published a series of standards addressing fluids for HDF. Bacteria- and endotoxin-retentive filters installed on the inlet dialysis fluid circuit are the key components of the online HDF safety system2). Two stages of reverse osmosis in series water with a resistivity in the range of 10 to 20 MΩ is needed and the water should be sterile and nonpyogenic4). European standards (ERA-EDTA) water are bacterial limits <100CFU/mL and endotoxin limits <0.25IU/mL. Ultrapure water should meet the conditions of bacterial limits <0.1CFU/mL and endotoxin limits <0.03 IU/mL5). The water quality should be monitored every 1-3 month.
HDF can be applied in those conditions listed below.
1) Inadequate clearance on standard therapy such as uncontrolled hyperphosphatemia
2) Suffers of dialysis induced complication such as β2-microglobulin amyloidosis, MIA syndrome and intradialytic complications
3) Young patients before occurring long-term complications which can affect patients lifestyle
4) Patients with a lifespan >5 years
5) Preservation of residual renal function for longer period
6) Others
Mode of HDF is defined according to the replacement site of extra ultrapure water as substitute volume. In the post-dilution HDF, the replacement fluid is infused downstream of the dialyzer6). The replacement fluid is infused up-stream of the dialyzer in the pre-dilution HDF7). Mixed dilution HDF is a combined type of pre and post-dilution HDF. For mid-dilution HDF, which the replacement fluid is infused part-way down the blood pathway, a specially designed dialyzers or systems are needed8).
Blood flow and filtration fraction (FF) are the main factors related to total convection volume. High blood flow of above 350mL/min and FF of below 25% are preferred. Clinical benefits include, low rate of hospitalization, easy corrections of anemia and hyperphosphatemia. Also, rising survival rates were observed in many observational and randomized control studies such as the European DOPPS (Dialysis Outcomes and Practice Patterns Study), RISCAVID ('RISchio CArdiovascolare nei pazientiafferenti all'Area Vasta In Dialisi'), EUCLID (European CLInical Database), CONTRAST (CONvectiveTRAnsport Study), Turkish on-HDF trial and ESH-OL(Estudio de Supervivencia de Hemodiafiltracion On-Line) which used high convection volume. It appears that these benefits are consistently associated with a convection volume above 20 to 22 L per treatment. Recommendations to obtain the optimal HDF dose are listed on Table 1.
In Korea, conventional hemodialysis has been done widely in many dialysis facilities rather than HDF. As we discussed above, HDF has many clinical benefits. However, there are some obstacles for doing HDF consistently. New HDF machines, ultrapure water quality, trained staff and additional costs are needed for HDF. Although these limitations currently exist, we can do HDF with success. We have HDF machines already since recently developed hemodialysis machines can do HDF generally. As most dialysis methods using high flux membranes not only HDF needs ultrapure water, installation of ultrapure water system from the setting of dialysis facilities can save the extra costs. The problem with training doctors and nurses may be solved with dialysis companies and educational institutes. Additional cost more than conventional hemodialysis should be overcome in several ways. According to the survey in Italy, direct medical costs such as drugs, equipment, tests and hospitalizations were higher for Ol-HDF by a scale of 20-30% than conventional hemodialysis and were revealed the difference in costs between two modalities mainly depends on the materials and lab tests used for water9). For example, if the blood line with a cuvette is used for blood volume monitor, Ol-HDF is more expensive in terms of consumables used per treatment. However, these problems can be solved using standard blood line and medical reimbursement system. Unfortunately, there are no incentives for Ol-HDF in Korea yet. Medical reimbursement policy differs from country. Slovenia, Slovakia, Czech Republic and Greece have a special reimbursement for HDF therapies without restrictions, however the percentage of HDF is limited in order to keep the overall dialysis cost under control. Serbia, Spain, Italy, United Kingdom and Russian have a special reimbursement for HDF with restrictions according to the type of provider (public or individual) and the reimbursement rate vary. In the majority of European countries like Switzerland, Belgium, Austria, Netherlands, France and Germany, no extra reimbursement is provided for this9). Also, United States and Ontario in Canada have no incentive in the reimbursement10). Reimbursements for dialysis of 7 countries are listed on Table 2. On the other hand, Ol-HDF was officially approved for reimbursement by the Japanese health insurance system in April 2012. After the approval, the number of Ol-HDF patients dramatically increased11). However, we cannot deny the positive effects of HDF which can outweigh the additional costs in the future. There will be clear-cut indications after more randomized control studies proving evidence of HDF superiority to HD. HDF can be done effectively and economically with selective patients in spite of the coexisting limitations.
References
1. Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003; 63:1934–1943. PMID: 12675874.
2. Tattersall JE, Ward RA; EUDIAL group. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant. 2013; 28:542–550. PMID: 23345621.

3. Maduell F. Is There an 'Optimal Dose' of Hemodiafiltration? Blood Purif. 2015; 40:17–23. PMID: 26344509.

4. Canaud B, Chenine L, Henriet D, Leray H. Online hemodiafiltration: a multipurpose therapy for improving quality of renal replacement therapy. 2008.
5. Canaud B, Lertdumrongluk P. Ultrapure Dialysis Fluid: A New Standard for Contemporary hemodialysis. Nephrourol Mon. 2012; 4:519. PMID: 23573478.

6. Ledebo I, Blankestijn PJ. Haemodiafiltration-optimal efficiency and safety. NDT plus. 2010; 3:8–16. PMID: 20090878.

7. Canaud B, Lévesque R, Krieter D, et al. On-line hemodiafiltration as routine treatment of end-stage renal failure: why pre-or mixed dilution mode is necessary in on-line hemodiafiltration today? Blood Purif. 2004; 22:40–48. PMID: 15655323.
8. Krieter DH, Collins G, Summerton J, Spence E, Moragues HL, Canaud B. Mid-dilution on-line haemodiafiltration in a standard dialyser configuration. Nephrol Dial Transplant. 2005; 20:155–160. PMID: 15522903.

9. Del Vecchio M, Giordana G, Pedrini L, Marcelli D, Sisti N. Elements for economic evaluation on online Hemodiafiltration (ol-HDF) versus standard Haemodialysis to treat patients with End-Stage Renal Disease (ESRD). Ital J Public Health. 2012; 9.
10. Vanholder R, Davenport A, Hannedouche T, et al. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol. 2012; 23:1291–1298. PMID: 22677554.

11. Akizawa T, Koiwa F. Clinical expectation of online hemodiafiltration: A Japanese Perspective. Blood Purif. 2015; 40:12–16. PMID: 26344508.

Table 2
Reimbursement adjustments for alternative nonstandard dialysis strategies or specific patient groups
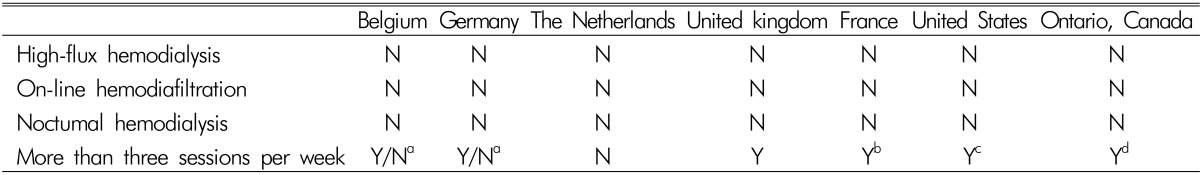
N, no incentive or disincentive
aY stands for hospital hemodialysis; N stands for other options
bAny fourth session per week
cA fourth session is reimbursed if medically justified
dIn-home hemodialysis is $385 for three times per week but $760 for five to six times per week
Ref. J Am SocNephrol 2012;23:1293




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download