Abstract
Background
The relationship between abdominal obesity (AO) and mortality in peritoneal dialysis (PD) patients is controversial.
Methods
The prevalence of AO in 84 PD patients was assessed in a cross-section manner and followed up for 9 years at a single center. AO was defined as a waist circumference (WC) of more than 90 cm in males or more than 80 cm in females. The patients were classified as either with AO(AO group) or without AO(nAO group).
Results
The AO group was older, contained more diabetics, more females, and had higher Charlson comorbidity index (aCCI) scores, BMI, and triglyceride and lower serum creatinine than the non-AO subjects. The follow-up duration was 53.2±34.4 months. At the end of the follow-up, eighteen patients (21.4%) were dead; 9 died of cardiovascular causes. The five year survival rate was 40.8%. Kaplan-Meier analysis revealed that both all-cause and cardiovascular-cause mortalities were similar in the AO and nAO groups. Multivariate analysis revealed the presence of AO not to be an independent risk factor of all-cause and cardiovascular-cause mortality.
In ESRD patients, there have been significant increases in the prevalence of obesity among incident patients both in the USA and Europe12). In addition, AO appears to be problematic in peritoneal dialysis (PD) patients3). PD solutions contain high concentrations of glucose (>200 mmol/L)4). On average, 65% of intraperitoneally-administered glucose is absorbed during a 4 hour dwell, irrespective of the initial dialysate glucose concentration5). Therefore, PD patients appear to become more abdominally obese than HD patients6).
AO appears to be a risk factor for death in ESRD patients on hemodialysis (HD), as in the general population789). In PD patients, several studies have also reported AO to be a risk factor for mortality101112). Despite this, the follow-up periods of those studies were relatively short except for Lee et al.101112).
This study examined the effects of AO on the survival of PD patients. To accomplish this, the prevalence of AO in PD patients was assessed cross-sectional manner and followed up for 9 years at a single center.
This retrospective study was performed on ESRD patients on CAPD who had been followed up at the outpatient PD clinic, Inha University Hospital in Incheon, Korea. The inclusion criteria were 152 patients, who had received a cross-sectional nutritional assessment between September 2003 and March 2004. The CAPD system was a twin-bag. The CAPD prescription was 4×2 liter exchanges. In the case of a dialysis inadequacy, 5 exchanges were prescribed. The adequacy of CAPD was evaluated by measuring the weekly total Kt/V for urea and the creatinine clearance13).
The anthropometric measurements and nutritional assessments were performed by a well-trained dietitian at the PD clinic on the days they had visited for a monthly laboratory examination. Dialysate was drained from the abdomen. The height was then measured to the nearest 0.1 cm using a linear height scale. The body weight (BW) and fat mass were measured using the eight-point tactile electrode impedance method (Inbody 3.0; Biospace, Seoul, Korea). The body mass index (BMI) was calculated as the weight (in kilograms) divided by the square of the height (in meters). The waist circumference (WC) was measured at the umbilicus level at the end of expiration using a flexible plastic tape measure. The mean of two measures were used for analysis. AO was defined as a WC greater than 90 cm in males or greater than 80 cm in females14). The subjective global assessment (SGA) was used to evaluate the nutritional status15).
The patients were followed up until their death, kidney transplantation, transfer to HD or other hospital, or July 31, 2012. Their demographic, clinical and biochemical data was collected from the medical records. LDL-cholesterol was calculated by Friedewald equation16). Comorbidity was assessed using the age-adjusted Charlson comorbidity index (aCCI) score1718). Patients on CAPD of more than 2 years (n=46), lacking data of the WC(n=10) and had a follow-up duration of less than 6 months (n=10) after a nutritional assessment were excluded. Overall, 84 patients were included in the final analysis.
The primary outcome was the all-cause mortality. The secondary outcome was the cardiovascular-cause mortality from fatal cardiovascular events, which were defined as death from cardiovascular causes and stroke.
Statistical analysis was performed using SPSS for Windows Version 19.0 (SPSS Inc., Chicago, IL). The data is expressed as the mean±SD. The patients were classified as either with AO(AO group) or without AO(nAO group). The continuous variables between 2 groups were compared using an independent t-test. The nominal variables were assessed using a Chi-square test. The overall patient survival was estimated using the Kaplan-Meier method and a comparison of the outcomes were based on the log rank test. Kidney transplantation, technical failure and transfer to other hospitals were censored observations for patient survival analysis. Technical failure was defined as transfer to HD due to peritonitis, ultrafiltration failure, or mechanical problems. Mortality was analyzed using the multivariate Cox's proportional hazard model, in which all the significant variables from univariate analysis were included (in a backward manner). p<0.05 were considered significant.
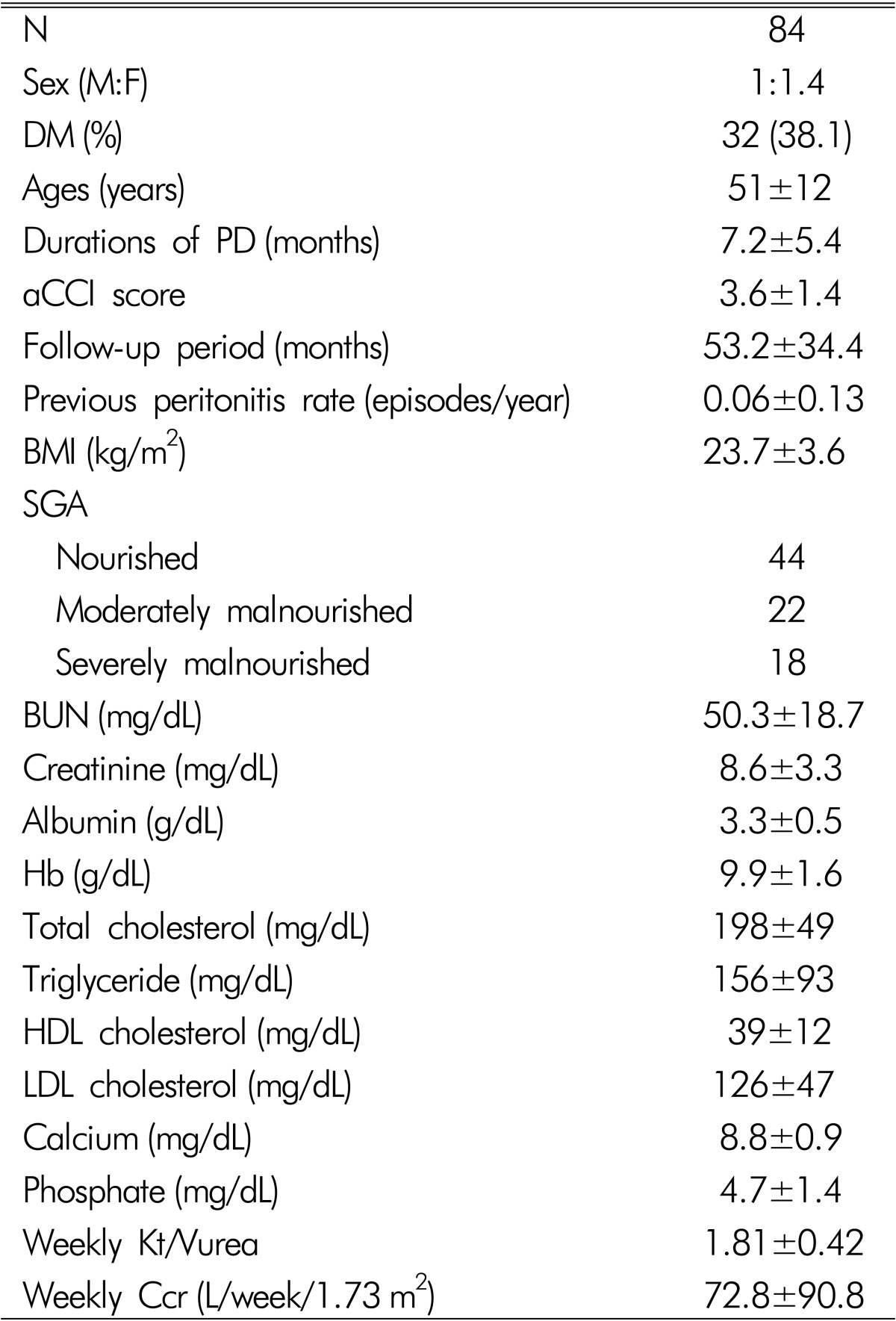
Table 1 lists the baseline characteristics of 84 subjects. The mean age of the subjects was 51±12 years. The male to female ratio was 1:1.4. Thirty two patients (38.1%) were diabetics. The previous durations of CAPD were 7.2±5.4 months. The aCCI score was 3.6±1.4. The BMI was 23.7±3.6 (range 17.2-33.6) kg/m2. The previous peritonitis rate was 0.06±0.13/year. According to the SGA, 44 were nourished, 22 were moderately malnourished and 18 were severely malnourished. The hemoglobin, BUN, serum creatinine, albumin, total cholesterol, triglyceride, LDL cholesterol, total calcium, and phosphate levels were 9.9±1.6 g/dL, 50.3±18.7, 8.6±3.3mg/dL, 3.3±0.5 g/dL, 198±49, 156±93, 39±12, 8.8±0.9, and 4.7±1.4mg/dL, respectively. The CAPD adequacies were examined in 53 patients. The weekly Kt/Vurea and creatinine clearance were 1.84±0.42 and 76.8±90.8 L/week/1.73m2, respectively.
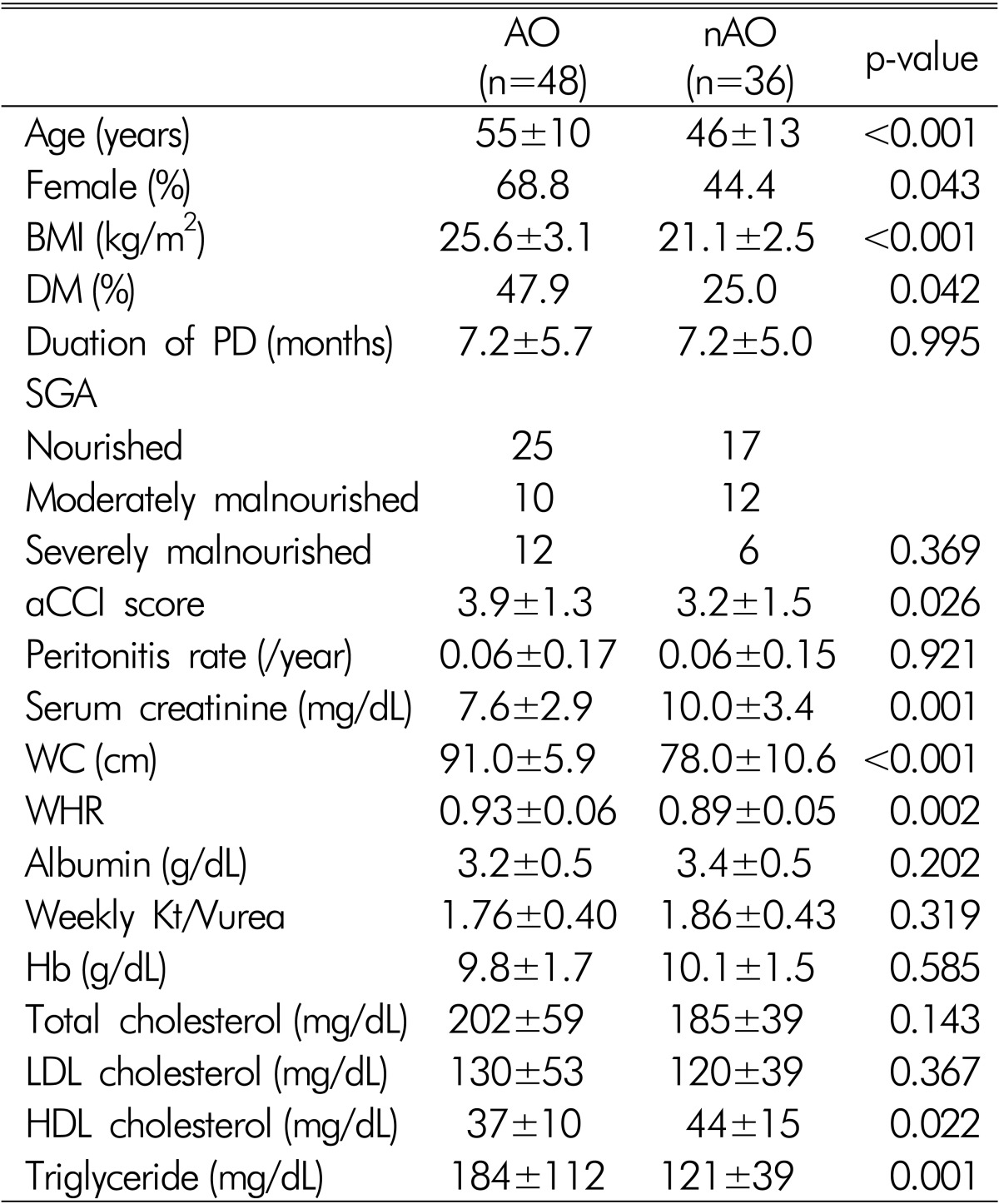
Among the 84 patients, the number of AO subjects was 48 (57.1%). The AO group was older, contained more diabetics, more females, and had higher aCCI scores, BMI and triglyceride, and lower serum creatinine concentration than the nAO group (Table 2). The previous duration of CAPD, nutritional status and peritonitis rates were similar in the AO and nAO groups.
The follow-up duration was 53.2±34.4 months. At the end of the follow-up, eighteen (21.4%) were dead. Nine died of cardiovascular causes. The five year survival rate was 40.8%. Kaplan-Meier analysis showed that both all-cause (p=0.767) and cardiovascular-cause mortalities (p=0.983) were similar in the AO and nAO groups (Fig. 1).
In multivariate analysis adjusted for sex, aCCI score, serum creatinine, triglyceride, HDL-cholesterol, group (AO vs. nAO), and BMI, aCCI score, and serum creatinine were found to be independent risk factors of all-cause and cardiovascular-cause mortality (Table 3).
In this study, the presence of AO was not a risk factor for mortality in CAPD patients. The all-cause and cardiovascular-cause mortalities were similar in the patients with and without abdominal obesity. In the general population, obesity is a risk factor for CVD and is associated with increased mortality1920). On the other hand, there has been some controversy as to whether obesity is a risk factor for death in ESRD patients because several survival studies have suggested that a higher BMI is associated with improved survival2122). Currently, the fat distribution has different metabolic effects and abdominal fat is associated more closely with mortality than the total or peripheral fat23242526). Insulin resistance is induced by fat deposited intracellularly, and by proinflammatory cytokines, such as interleukins 1 and 6 and tumor necrosis factor α, secreted by adipose tissue2728). AO accentuates the problem, possibly because of the unusually high influx of portal fatty acids, cytokines and hormones into the liver from the omental adipocytes that are normally almost devoid of fat27). The effect of cytokines on the peripheral tissues with increased intracellular lipid also includes lower cellular insulin sensitivity27). The surge in lipids promotes the proliferation of the vasa vasorum of the arterial media, and apoptosis by the medial macrophages, with the further release of cytokines, resulting in hypertension, cardiovascular disease, diabetes mellitus, and others27).
AO appears to be a risk factor for death in ESRD on HD patients, as in the general population. Several studies have reported that AO is associated with mortality in ESRD on HD patients789). The PD patients may be at higher risk of developing AO compared to HD patients due to the use of glucose-based dialysis solutions10). The majority of patients experienced significant weight gain after the initiation of PD29). Visceral obesity was reported to be more common in PD patients than HD patients6). PD patients showed a 23% increase in the intra-abdominal fat area after initiating PD, despite the lack of a significant increase in weight or proportion of total fat mass30). WHR independently predicted mortality and CV events in 22 PD patients10). The WC and waist/hip ratio (WHR) were risk factors of all-cause and CV mortality in 537 ESRD patients9). Nevertheless, it was not known how many were undergoing peritoneal dialysis. Stolic et al. that the WC and BMI were predictors of death in PD patients11). In a prospective observational study, the sagittal abdominal diameter, which is a surrogate index of abdominal obesity, was also an independent predictor of mortality in 418 incident PD patients12).
In this study, the prevalent PD patients were followed up for more than 9 years after a cross-sectional assessment and the results were in contrast to other studies. Multivariate analysis revealed the presence of AO was not an independent risk factor for all-cause or cardiovascular-cause mortalities. Several possible causes of these differences can be speculated. The small number of subjects and short follow-up period used to evaluate the risk of death by AO are some reasons. Some studies, however, have reported lower number of subjects or shorter follow-up period than this study1011). Some studies support our results3132) Navaneethan et al. also reported that the presence of metabolic syndrome was not associated with mortality in patients with stages 3 and 4 chronic kidney disease (CKD)31). On the other hand, they used the BMI instead of the WC and the subjects were CKD patients not on dialysis. In 329 Chinese PD patients, the presence of metabolic syndrome was not a risk factor of death32). In a prospective study of 183 Chinese PD patients, abdominal fat was not associated with clinical outcomes such as CVD events or mortality33). In another prospective observational study with 117 Korean PD patients, increased abdominal fat at PD initiation was not a risk factor for poor outcome34).
The polymorphism in uncoupling protein 2 (UCP2) was reported to be associated with fat tissue accumulation during PD3536). There might be a racial difference in UCP2 gene polymorphism between Asians and Caucasians because no association between AO and mortality in PD patients were mostly reported from Asian subjects.
This study had several limitations. First, the AO was not measured using objective methods, such as computed tomography. Instead, the WC was used as an index of AO. Many indices, such as the WC, WHR, waist-height ratio, sagittal abdominal diameter, index of central obesity, and conicity index, were developed to express AO 263738394041). Among them, the WC is considered to be a simple anthropometric index of AO38). Furthermore, it was suggested that the WC alone could replace the WHR and BMI as a single risk factor for the all-cause mortality42). On the other hand, it showed low sensitivity when used as a single tool to identify older patients with either generalized or AO43). Furthermore, in PD patients, the WC may not reliably reflect the abdominal visceral fat content because the WC is affected by the presence of a catheter in situ, by lax skin conditions after repeated distention of the abdomen by PD fluid, and by potential residual PD fluid in the abdominal cavity44). The WC, however, was reported to be a reliable marker of AO in PD patients45), and was also associated with AO in CKD patients4546). Second, the subjects of this study were prevalent PD patients, not incident patients. Previous durations of CAPD were 21.5±22.3 (range 1-111) months. The absorption of 100-200 g of glucose per day from the conventional CAPD solution may promote obesity, glucose intolerance, insulin resistance, and the atherogenic lipid profile4). Therefore, patients with a longer duration of CAPD might have more AO. To reduce the effect of previous CAPD duration, in this study, the inclusion criteria was limited to dialysis vintage of less than 2 years and there were no differences in the previous duration of CAPD between the AO and nAO groups. Third, the effect of a bioincompatible solution was not considered. At the time of the cross-sectional nutritional assessment, the patients used a conventional CAPD solution because biocompatible solutions have been in use since the mid-2000s. Fourth, the peritoneal membrane transport characteristics were not included because at the initiation of this study, it was not regularly measured for all patients. Early studies found that the peritoneal membrane transport characteristics were important determinants of mortality in PD patients4748). Currently, it is controversial as to whether higher peritoneal transport characteristics at the start of peritoneal dialysis are associated with higher mortality4950). Fifth, the National Cholesterol Education Program(NECP) definition for AO is one of the most widely used definitions in the general population because of its simplicity and clinical relevance351). On the other hand, in this study, the definition of AO for the WC followed the International Diabetes Federation (IDF) criteria in 200514) because it considers ethnicity in its definition3). Sixth, there appeared to be selection bias of the subjects and the results of this study seemed to have low statistical power because it was a retrospective observational study at a single center. Despite this, the subjects were cared for by the same nephrologists for the follow-up period. Therefore, the results are less influenced by other factors, such as the center effect.
In conclusion, AO itself might not be a risk factor for mortality in PD patients. Nevertheless, further prospective studies with a larger number of patients will be needed to prove this.
Acknowledgements
The authors wish to thank Jinkyung Park and Professor SookMee Son, Department of Food and Nutrition, Catholic University, Buchon, Republic of Korea, for their help in the cross-sectional nutritional assessment during 2003 and 2004.
References
1. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case control study. Lancet. 2004; 364:937–952. PMID: 15364185.
2. Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006; 17:1453–1459. PMID: 16597682.

3. Zoccali C. The obesity epidemics in ESRD: from wasting to waist? Nephrol Dial Transplant. 2009; 24:376–380. PMID: 18952700.

4. Li PK, Kwan BC, Szeto CC, Ko GT. Metabolic syndrome in peritoneal dialysis patients. NDT Plus. 2008; 1:206–214. PMID: 25983884.

5. Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol. 2010; 6:451–460. PMID: 20567248.

6. Krediet RT, Boeschoten EW, Zuyderhoudt FM, Arisz L. The relationship between peritoneal glucose absorption and body fluid loss by ultrafiltration during continuous ambulatory peritoneal dialysis. Clin Nephrol. 1987; 27:51–55. PMID: 3829478.
7. Ku YM, Kim YS, Yoon SA, et al. Comparison of prevalence of visceral obesity between hemodialysis and peritoneal dialysis patients. Korean J Nephrol. 2008; 27:458–464.
8. Cordeiro AC, Qureshi AR, Stenvinkel P, et al. Abdominal fat deposition is associated with increased inflammation, protein-energy wasting and worse outcome in patients undergoing haemodialysis. Nephrol Dial Transplant. 2010; 25:562–568. PMID: 19762603.

9. Wu CC, Liou HH, Su PF, et al. Abdominal obesity is the most significant metabolic syndrome component predictive of cardiovascular events in chronic hemodialysis patients. Nephrol Dial Transplant. 2011; 26:3689–3695. PMID: 21357211.

10. Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009; 53:1265–1272. PMID: 19358939.

11. Su WS, Clase CM, Brimble KS, Margetts PJ, Wilkieson TJ, Gangji AS. Waist-to-Hip Ratio, Cardiovascular Outcomes, and Death in Peritoneal Dialysis Patients. Int J Nephrol. 2010; 2010:831243. PMID: 21188241.

12. Stolic R, Trajkovic G, Jovanovic A, et al. Association of metabolic changes with mortality of patients treated by peritoneal dialysis or hemodialysis. Ren Fail. 2010; 32:778–783. PMID: 20662689.

13. Lee MJ, Shin DH, Kim SJ, et al. Sagittal abdominal diameter is an independent predictor of all-cause and cardiovascular mortality in incident peritoneal dialysis patients. PLoS One. 2013; 8:e77082. PMID: 24167560.

14. Nolph KD, Moore HL, Prowant B, et al. Cross sectional assessment of weekly urea and creatinine clearances and indices of nutrition in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1993; 13:178–183. PMID: 8369345.

15. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005; 366:1059–1062. PMID: 16182882.
16. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.

17. Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987; 11:8–13. PMID: 3820522.

18. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994; 47:1245–1251. PMID: 7722560.

19. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999; 341:1097–1105. PMID: 10511607.

20. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006; 355:763–778. PMID: 16926275.

21. Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004; 4:94. PMID: 15610554.

22. Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005; 46:489–500. PMID: 16129211.

23. Kwon HS, Park YM, Lee HJ, et al. Prevalence and clinical characteristics of the metabolic syndrome in middleaged Korean adults. Korean J Intern Med. 2005; 20:310–316. PMID: 16491829.

24. Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008; 359:2105–2120. PMID: 19005195.

25. Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006; 84:449–460. PMID: 16895897.

26. Carmienke S, Freitag MH, Pischon T, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013; 67:573–585. PMID: 23511854.

28. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008; 9:367–377. PMID: 18401346.

29. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003; 63:793–808. PMID: 12631061.

30. Diaz-Buxo JA, Burgess WP. Is weight gain inevitable in most chronic peritoneal dialysis patients? Adv Perit Dial. 1992; 8:334–339. PMID: 1361818.
31. Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol. 2013; 8:945–952. PMID: 23411425.

32. Szeto CC, Kwan BCH, Chow KM, et al. Metabolic syndrome in peritoneal dialysis patients: choice of diagnostic criteria and prognostic implications. Clin J Am Soc Nephrol. 2014; 9:779–787. PMID: 24458080.

33. Huang JW, Yang CY, Wu HY, et al. Metabolic syndrome and abdominal fat are associated with inflammation, but not with clinical outcomes, in peritoneal dialysis patients. Cardiovasc Diabetol. 2013; 12:86. PMID: 23758640.

34. Choi SJ, Kim EJ, Park MY, Kim JK, Hwang SD. Does body fat mass define survival in patients starting peritoneal dialysis? Perit Dial Int. 2014; 34:376–382. PMID: 23378474.

35. Nordfors L, Heimburger O, Lonnqvist F, et al. Fat tissue accumulation during peritoneal dialysis is associated with a polymorphism in a uncoupling protein 2. Kidney Int. 2000; 57:1713–1719. PMID: 10760107.
36. Wang X, Axelsson J, Nordfors L, et al. Changes in fat mass after initiation of maintenance dialysis is influenced by the uncoupling protein 2 exon 8 insertion/deletion polymorphism. Nephrol Dial Transplant. 2007; 22:196–202. PMID: 16982633.

37. Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991; 44:955–956. PMID: 1890438.

38. Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994; 73:460–468. PMID: 8141087.

39. Parikh RM, Joshi SR, Pandia K. Index of central obesity is better than waist circumference in defining metabolic syndrome. Metab Syndr Relat Disord. 2009; 7:525–527. PMID: 19558273.

40. Iribarren C, Darbinian JA, Lo JC, Fireman BH, Go AS. Value of the sagittal abdominal diameter in coronary heart disease risk assessment: cohort study in a large, multiethnic population. Am J Epidemiol. 2006; 164:1150–1159. PMID: 17041127.

41. WHO. Waist Circumference and Waist-Hip Ratio: report of a WHO expert consultation. Geneva: World Health Organization (WHO);2008.
42. Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur J Clin Nutr. 2010; 64:35–41. PMID: 19639001.

43. Cockcroft A, Gooch C, Ellinghouse C, Johnston M, Michie S. Evaluation of a programme of health measurements and advice among hospital staff. Occup Med (Lond). 1994; 44:70–76. PMID: 8032035.

44. Li PK, Kwan BC, Ko GT, Chow KM, Leung CB, Szeto CC. Treatment of metabolic syndrome in peritoneal dialysis patients. Perit Dial Int. 2009; 292:S149–S152. PMID: 19270205.

45. Bazanelli AP, Kamimura MA, Manfredi SR, Draibe SA, Cuppari L. Usefulness of waist circumference as a marker of abdominal adiposity in peritoneal dialysis: a cross-sectional and prospective analysis. Nephrol Dial Transplant. 2012; 27:790–795. PMID: 21948862.

46. Sanches FM, Avesani CM, Kamimura MA, et al. Waist circumference and visceral fat in CKD: a cross-sectional study. Am J Kidney Dis. 2008; 52:66–73. PMID: 18440683.

47. Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998; 9:1285–1292. PMID: 9644640.

48. Wang T, Heimburger O, Waniewski J, Bergstrom J, Lindholm B. Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant. 1998; 13:1242–1249. PMID: 9623561.

49. Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006; 17:271–278. PMID: 16306167.

50. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002; 13:1307–1320. PMID: 11961019.

51. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program(NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. PMID: 11368702.
Fig. 1
Kaplan-Meier curve for (A) All-cause and (B) Cardiovascular-cause mortalities according to abdominal obesity.
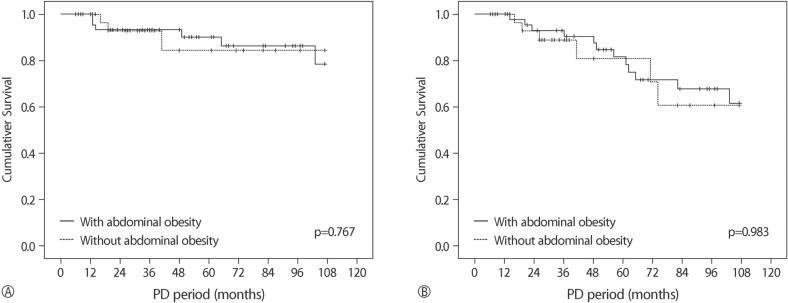
Table 3
Risk factors for all-cause and cardiovascular-cause mortalities
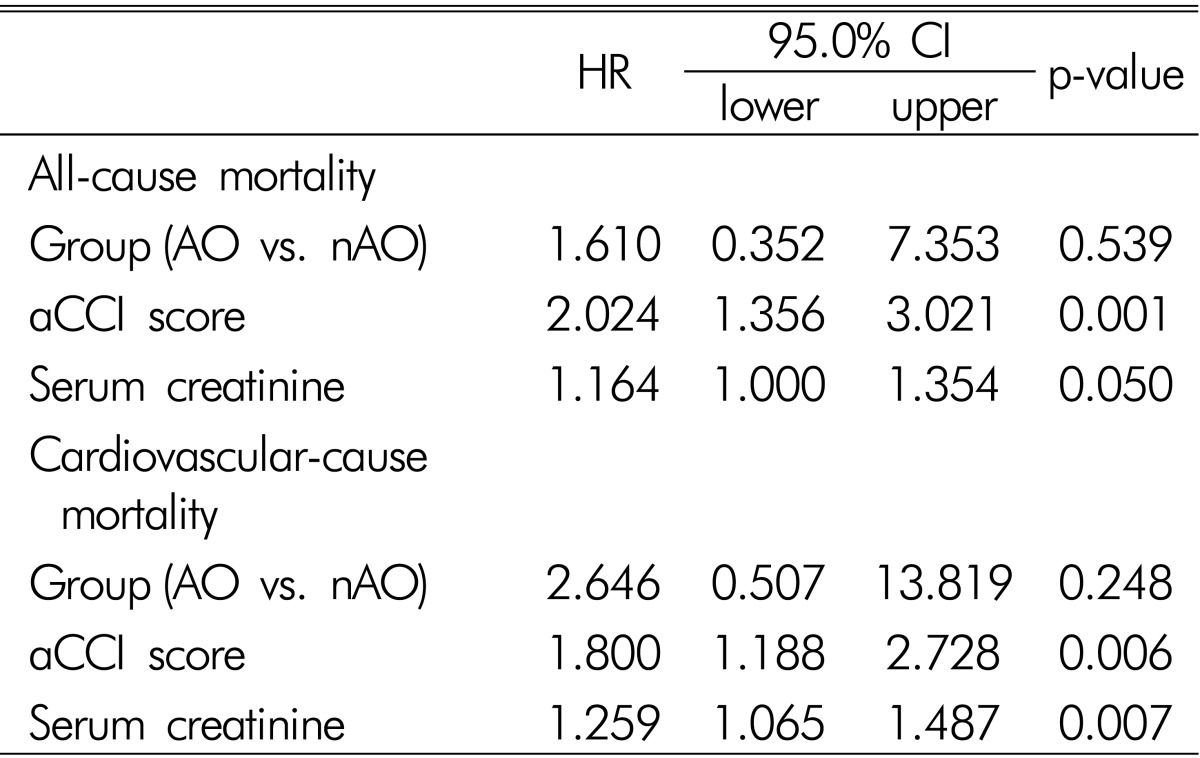
Results showed as HR and 95% CI (confidence interval), from Cox proportional hazard models. aCCI: age-adjusted Charlson comorbidity index, AO: abdominal obesity, nAO: no abdominal obesity.
Adjusted for aCCI, BMI (body mass index), sex, serum creatinine, triglyceride, HDL-cholesterol, and group (AO vs. nAO).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download