This article has been
cited by other articles in ScienceCentral.
Abstract
Current practice guidelines recommend alkali therapy in patients with chronic kidney disease (CKD) and metabolic acidosis to prevent complications. This study aims to investigate the effect of oral sodium bicarbonate supplementation on the progression of renal function and nutritional indices in patients with predialysis advanced CKD. Forty patients with predialysis stage 5 CKD(estimated glomerular filtration rate, eGFR <15mL/min per 1.73m2) and 40 patients with stage 4 CKD (eGFR 15 to 30mL/min per 1.73m2) who had a total CO2 less than 22mEq/L were assigned into the bicarbonate treatment group or control group for 12 months. In stage 4 CKD, there were significant differences in the changes of eGFR during the study between the treatment group and the control group (-2.30±4.49 versus -6.58±6.32mL/min/1.73m2, p<0.05). However, in stage 5 CKD, there were no significant differences in the change of eGFR during the study between the two groups (-2.10±2.06 versus -3.23±1.95mL/min/1.73 m2).There were no significant differences in the changes of nutritional indices such as albumin, prealbumin, transferrin, total lymphocyte count (TLC), and Ondodera's prognostic nutritional index (OPNI) during the study between the two groups. In stage 5 CKD, there were significant differences in the changes of TLC and OPNI between the two groups.
In conclusion, our results demonstrate that bicarbonate supplementation slows the rate of decline of renal function in stage 4 CKD and improves nutritional indices in stage 5 CKD. Alkali therapy in advanced CKD may have beneficial effect on renal function and malnutrition.
Keywords: Chronic kidney disease, Acidosis, Bicarbonate, Nutrition assessment
Introduction
Chronic metabolic acidosis is a common complication of advanced chronic kidney disease (CKD), especially among patients with an estimated glomerular filtration rate (eGFR) lower than 25mL/min/1.73m
2
1). It can have substantial adverse effects, including exacerbation of bone disease, increase of muscle degradation with muscle wasting, reduction of albumin synthesis, and possible acceleration of the progression of CKD
2).
Current practice guidelines recommend correcting the serum bicarbonate to greater than 22mEq/L to prevent potential adverse effects related to chronic metabolic acidosis in CKD
3). However, clinicians may be reluctant to prescribe the oral bicarbonate in advanced CKD patients with edema or uncontrolled hypertension. Although alkali therapy in CKD has been examined in some clinical trials
4,
5,
6,
7,
8,
9), information for the impact of bicarbonate supplementation in advanced CKD patients who have a high risk of severe malnutrition and edema is very limited.
We investigated the effect of oral bicarbonate supple mentation on renal function and nutritional indices in predialysis advanced CKD patients.We compared the effect of bicarbonate supplementation in patients with stage 4 and predialysis stage 5 CKD.
Methods
Forty patients with stage 5 CKD not receiving renal replacement therapy (eGFR <15mL/min per 1.73m2) and 40 patients with stage 4 CKD(eGFR 15 to 30mL/min per 1.73m2) who had total CO2 less than 22mEq/L were enrolled from outpatient clinics of Chungbuk National University Hospital. Patients were assigned to receive either oral sodium bicarbonate in the treatment group or standard care without alkali in the control group for 12 months. In the 40 patients with alkali treatment group, the dosage of sodium bicarbonate (Tasna®, HCO3 5.95 mEq/500mg) were started 1,000mg thrice daily and then were adjusted as necessary to maintain total CO2 level greater than 22mEq/L. We excluded patients with malignant disease, liver cirrhosis, infection, sepsis, and overt congestive heart failure. This study was approved by the Institutional Review Board of Chungbuk National University Hospital. Blood pressure, body weight, serum electrolyte, total CO2, creatinine, BUN, eGFR, calcium, phosphorous, intact PTH were assessed at baseline and 12 month of the study. The eGFR was calculated by the Modification of Diet in Renal Disease formula: eGFR (mL/min/1.73m2)=186×(serum creatinine)-1.154×(Age)-0.203× 0.742 (female).
Nutritional indices were assessed body mass index (BMI), mid-arm muscle circumference (MAMC), prealbumin, albumin, transferrin, total lymphocyte count (TLC), and Onodera's prognostic nutritional index (OPNI) at baseline and 12 month of study. BMI was derived using weight and height. MAMC was derived from the Bishop
10) formula using mid-arm circumference (MAC) and triceps skin-fold thickness (TSF):MAMC(cm)=MAC(cm)-TSF (mm)×0.314. TLC was calculated by multiplying the percentage of lymphocytes with the total white blood cell count. The OPNI was calculated based on the serum albumin and total lymphocyte count, using the following equation: OPNI=10×serum albumin (g/dL)+0.005×total lymphocyte count (/mm
3)
11).
SigmaPlot 12.0 for windows software was used for all statistical analysis (Systat Software Inc, San Jose, CA, USA). Values are expressed as mean±SD. The comparisons between baseline and 12 month were assessed by paired t-test, and comparisons between groups were assessed by unpaired t-test or Wilcox singed-rank test. A p-value of less than 0.05 was considered statistically significant.
Results
1. Patient characteristics
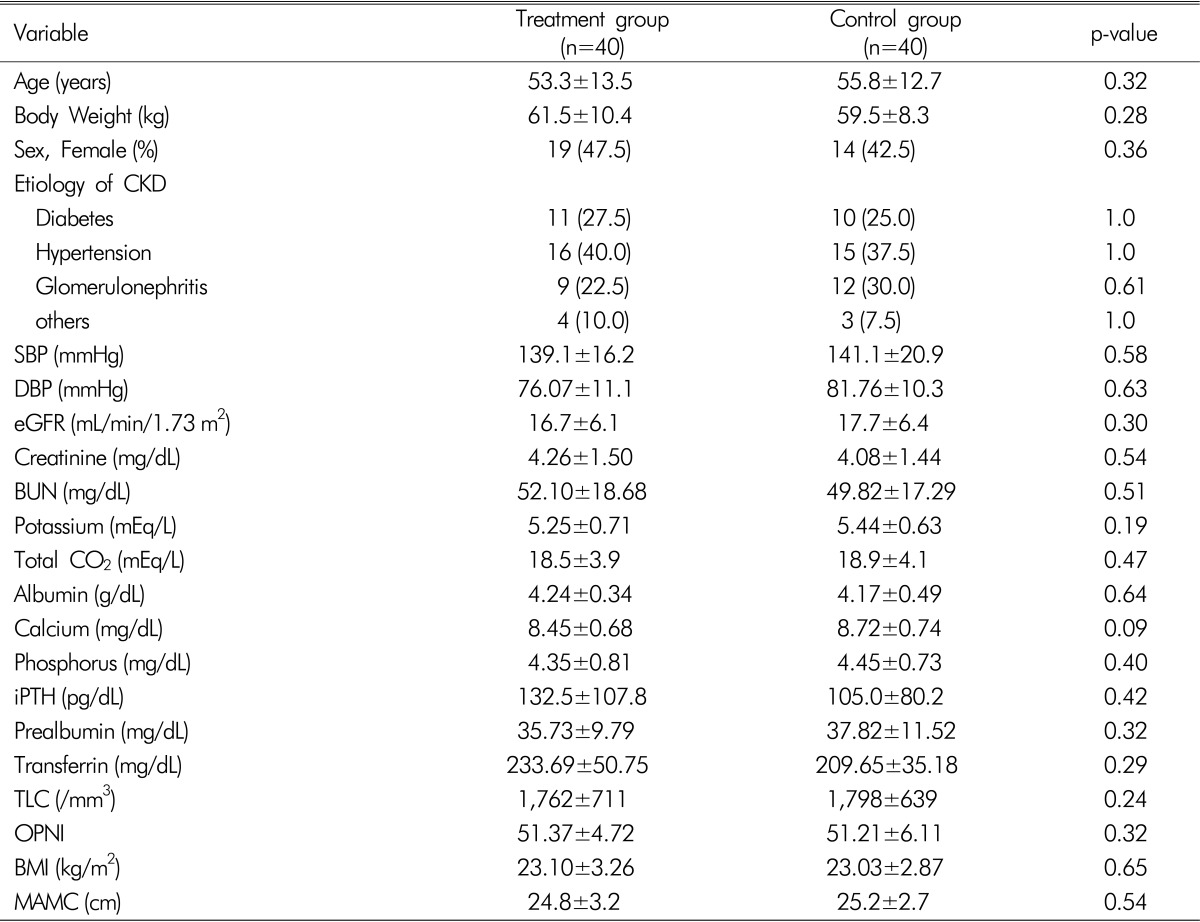
The baseline demographic, laboratory, and nutritional datas of the study group are shown in
Table 1. There were no significant differences in age, body weight, gender, primary causes of CKD and blood pressure. There were no significant differences in baseline eGFR, creatinine, BUN, potassium, total CO
2, albumin, calcium, phosphorus, intact PTH, prealbumin, transferrin, TLC, OPNI, BMI, and MAMC in the two groups.
2. Effect of oral bicarbonate supplementation on renal function
Of the 80 patients enrolled, 37 patients in the treatment group and 36 patients in the control group completed the study. In only stage 5 CKD, 3 patients in the treatment group and 4 patients in the control group dropped out because of progression to dialysis.
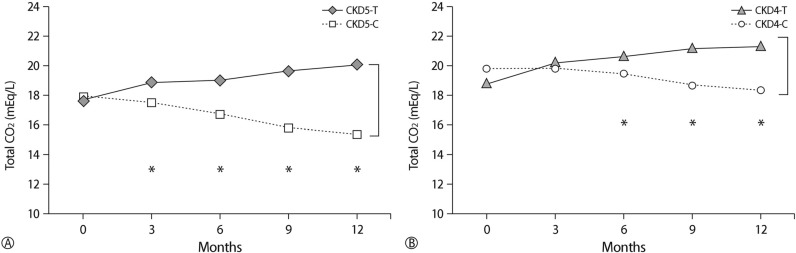
The mean dosage of oral sodium bicarbonate supplementation in the treatment group was 0.58±0.42mEq/kg. After alkali treatment, serum total CO
2 level was increased in stage 5 and stage 4 CKD(p<0.05,
Table 2). However, in the control group, serum total CO
2 level was decreased in stage 5 and stage 4 CKD(p<0.05,
Table 2). There was significant difference in changes of total CO
2 level in the treatment group compared with the control group during the study period (p<0.05,
Table 3,
Fig. 1).
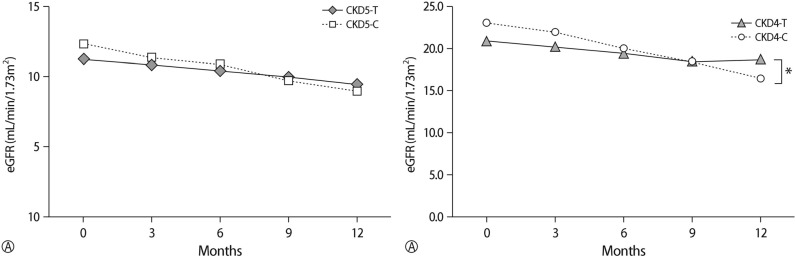
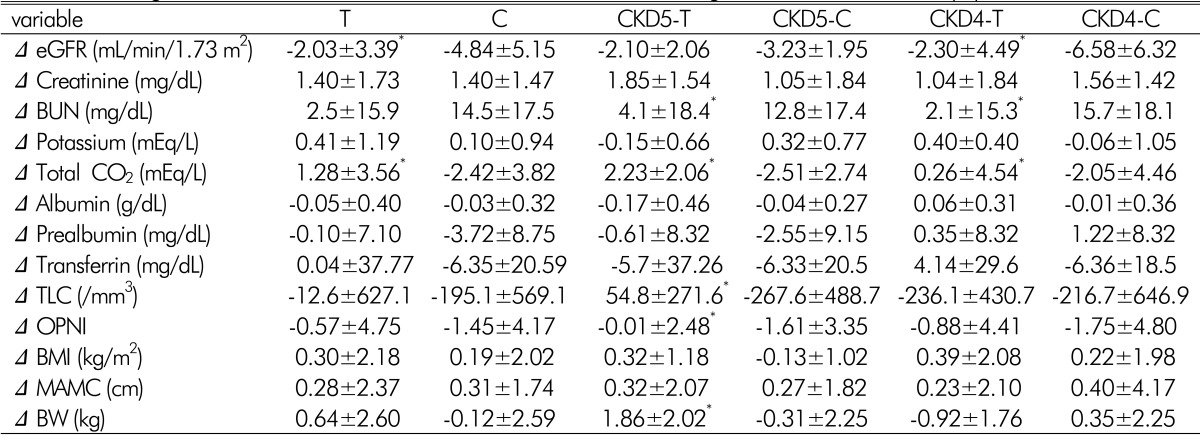
There was a significant decrease in eGFR between baseline and 12 months in both the treatment and control groups (p<0.05,
Table 2). The changes of eGFR in the treatment groups during the study period was significantly lower than in the control group (-2.03±3.39 mL/min/1.73m
2 in the treatment group vs. -4.84±5.15 mL/min/1.73m
2 in the control group, p<0.05,
Table 3). In stage 4 CKD, there was a significant difference in the changes of eGFR during the study between the treatment group and the control group (-2.30±4.49mL/min/1.73m
2 in the treatment group vs. -6.58±6.32mL/min/1.73m
2 in control group, p<0.05,
Table 3,
Fig. 2). However, in stage 5 CKD, there was no statistically significant difference in the change of eGFR during the study between the treatment group and the control group (
Table 3,
Fig. 2). There were no differences in changes of creatinine, BUN, potassium level in the treatment group compared with the control group during the study period. During the study period, serum calcium, phosphorous, intact PHT levels were similar between the treatment group and the control group (
Table 3).
3. Effect of oral bicarbonate supplementation on nutritional indices
There were no significant differences in albumin, preablumin, transferrin, BMI, and MAMC between baseline and 12 months in both treatment and control groups (
Table 2). However, there was a significant decrease in TLC and OPNI between baseline and 12 months in the control group of stage 5 CKD(p<0.05,
Table 2).
There was no significant difference in the changes of albumin, prealbumin, transferrin, TLC, OPNI during the study period between the treatment group and control group. The decline of TLC in the treatment group was lower than in the control group during the study period, which was not statistically significant (-12.6±627.1/mm
3 in the treatment group vs. -195.6±569.1/mm
3 in the control group, p=0.07,
Table 3). In stage 5 CKD, there was a significant difference in the changes of TLC and OPNI during the study between the treatment group and control group (p<0.05,
Table 3). However, in stage 4 CKD, there was no statistically significant difference in the change of TLC and OPNI during the study between the treatment group and control group (
Table 3).
4. The adverse events during the study period
Mean body weight was significantly increased after 12 months in the treatment group of stage 5 CKD(p<0.05,
Table 2). There was no significant difference in the changes of body weight during study period between the treatment group and control group (
Table 3). In stage 4 CKD, there was no statistically significant difference in the change of body weight during the study between the treatment group and control group. However, in stage 5 CKD, there was a significant difference in the changes of body weight during the study between the treatment group and control group (p<0.05,
Table 3).
There was no significant difference in the changes of systolic and diastolic blood pressure during the study between the treatment group and control group (3.4±16.3/0.7±11.5mmHg in the treatment group vs. -2.1±22.3/-1.8±12.3mmHg in the control group). There was no difference in the number and types of antihypertensive medications during the study between the two groups. All patients in stage 5 CKD was received loop diuretics at the end of the study. Loop diuretic use increased similarly by 75 and 82 % in the control and treatment group, respectively. Three patients in the treatment group and 4 patients in the control group required dialysis during the study period.
Discussion
Overt chronic metabolic acidosis in CKD patients develops after a drop in GFR to less than approximately 25mL/min/1.73m
2
1). The National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines recommend correcting the serum bicarbonate to greater than 22mEq/L to prevent potential adverse effects related to chronic metabolic acidosis, including bone disease, progression of CKD and malnutrition
1,
3). Although alkali therapy in CKD has been examined in some clinical trials
4,
5,
6,
7,
8,
9), information on the effects of bicarbonate supplementation in advanced CKD patients who have a high risk of severe malnutrition, uncontrolled hypertension and edema is very limited.
In a randomized, prospective study, de Brito-Ashurst et al.
5) suggested that sodium bicarbonate slowed the rate of creatinine clearance decline from 5.93 to 1.88mL/min per 1.73m
2/year in patients with stage 4 CKD. Phisitkul et al.
12) noted sodium citrate slowed the rate of decrease in eGFR in patients with hypertensive nephropathy with eGFR of 20 to 60mL/min/1.73m
2.
We compared the effects of oral bicarbonate supplementation on the progression of CKD and nutritional indices in predialysis stage 5 and stage 4 CKD. This study demonstrated that oral bicarbonate supplementation slowed the rate of decline of eGFR in stage 4 CKD. However, rate of decline of eGFR was similar between the treatment group and control group in stage 5 CKD. In this study, the annual decline of eGFR in stage 4 CKD is similar to the previously reported study
5,
12).
Malnutrition is a potential consequence of chronic metabolic acidosis
13). In advanced CKD, treatment of severe acidosis has produced improvements in anthropometric measures of lean body mass in some but not all reports
14,
15,
16,
17). Several small, short-term clinical trials, mainly of dialysis patients, suggested that correction of acidosis is associated with increased serum albumin and improved nutritional status
18). Uremic acidosis can increase skeletal muscle breakdown and diminish albumin synthesis, leading to muscle wasting and muscle weakness
1,
13). The catabolic state appears to be mediated by acidosis, acting in part by the increased release of cortisol and diminished release of insulin-like growth factor-I, leading to the loss of lean body mass and muscle weakness
19). These abnormalities in muscle function and/or albumin metabolism can be reversed by alkali therapy to correct the acidosis, including optimal correction of acidosis in patients undergoing chronic dialysis
16,
17,
18).
We were unable to demonstrate the beneficial effect of alkali therapy on serum albumin, and mid-arm muscle circumference in contrast to the previous studies
5). However, in this study, there were significant differences in the changes of TLC and OPNI during the study period between the treatment group and control group in stage 5 CKD, but not stage 4 CKD. Our study demonstrated that oral sodium bicarbonate supplementation for 12 months might improve the biochemical nutritional indices in stage 5 CKD.
TLC has been proposed as a prognostic factor
20). Malnutrition can induce a decrease in TLC and suppression of cellular immunity including delayed hypersensitivity reaction
21). Reddan et al.
22) showed that the lymphocyte count was associated with the nutritional status and mortality in dialysis patients. Onodera et al.
11) first reported the validity of the OPNI to predict the prognosis in gastrointestinal surgical patients.
In advanced CKD, the anticipated adverse effects of sodium bicarbonate supplementation were worsening hypertension and edema as a result of sodium retention. The adverse event of both groups were similar. In this study, after sodium bicarbonate supplementation, there was a tendency of weight gain and increased blood pressure in stage 5 CKD.
In meta-analysis, the sodium bicarbonate administered in the long-term trials had no adverse effect on blood pressure or cardiac function
23). Although alkali therapy is well tolerated in advanced CKD, potential complications of sodium bicarbonate, such as volume overload and worsened hypertension, need to be monitored carefully in stage 5 CKD.
This study has several strengths. To the best of our knowledge, the study is the first to examine the effect of bicarbonate supplementation in stage 5 CKD patients not on dialysis. In addition, various biochemical nutritional indices such as prealbumin, transferrin, TLC, and OPNI as well as albumin were used for assessment of nutritional status. Despite the strengths, there are limitations to this study. The small number of subjects in a single center study was not enough to compare the differences between groups. The serum total CO2 level did not reach greater than 22mEq/L during bicarbonate treatment.
Conclusion
Our results demonstrate that bicarbonate supplementation in advanced CKD with metabolic acidosis slows the rate of decline of renal function only in stage 4 CKD, but not stage 5 CKD. In addition, bicarbonate supplementation may have a beneficial effect on malnutrition in stage 5 CKD, but not stage 4 CKD. Alkali therapy in advanced CKD may have a beneficial effect on renal function and malnutrition, which may differ depending on the stage of CKD.
Acknowledgements
The paper was supported by the research grant of Chungbuk National University in 2012.
References
1. Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005; 45:978–993. PMID:
15957126.

2. Mitch WE. Influence of metabolic acidosis on nutrition. Am J Kidney Dis. 1997; 29:xlvi–xlviii. PMID:
9159296.

3. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000; 35:S1–S140. PMID:
10895784.
4. Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013; 8:714–720. PMID:
23393105.

5. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009; 20:2075–2084. PMID:
19608703.

6. Disthabanchong S, Treeruttanawanich A. Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol. 2010; 32:549–556. PMID:
21042013.

7. Gaggl M, Cejka D, Plischke M, et al. Effect of oral sodium bicarbonate supplementation on progression of chronic kidney disease in patients with chronic metabolic acidosis: study protocol for a randomized controlled trial (SoBic-Study). Trials. 2013; 14:196. PMID:
23826760.

8. Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013; 8:371–338. PMID:
23393104.

9. Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006; 28:1–5. PMID:
16526312.

10. Bishop CW, Bowen PE, Ritchey SJ. Norms for nutritional assessment of American adults by upper arm anthropometry. Am J Clin Nutr. 1981; 34:2530–2539. PMID:
6975563.

11. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984; 85:1001–1005. PMID:
6438478.
12. Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008; 73:192–199. PMID:
17978813.

13. Kraut JA, Madias NE. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol. 2011; 26:19–28. PMID:
20526632.

14. Bossola M, Giungi S, Tazza L, Luciani G. Long-term oral sodium bicarbonate supplementation does not improve serum albumin levels in hemodialysis patients. Nephron Clin Pract. 2007; 106:c51–c56. PMID:
17409769.

15. Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on albumin in chronic hemodialysis patients. Am J Kidney Dis. 1998; 31:35–40. PMID:
9428449.

16. Stein A, Moorhouse J, Iles-Smith H, et al. Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int. 1997; 52:1089–1095. PMID:
9328950.

17. Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: a randomized placebo-control trial. J Am Soc Nephrol. 2003; 14:2119–2126. PMID:
12874466.

18. Movilli E, Viola BF, Camerini C, Mazzola G, Cancarini GC. Correction of metabolic acidosis on serum albumin and protein catabolism in hemodialysis patients. J Ren Nutr. 2009; 19:172–177. PMID:
19218045.

19. Yaqoob MM. Treatment of acidosis in CKD. Clin J Am Soc Nephrol. 2013; 8:342–343. PMID:
23371954.

20. Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition. 2000; 16:131–140. PMID:
10696638.

21. Bistrian BR, Blackburn GL, Scrimshaw NS, Flatt JP. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr. 1975; 28:1148–1155. PMID:
810018.

22. Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003; 18:1167–1173. PMID:
12748351.

23. Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol. 2012; 35:540–547. PMID:
22653322.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download