Abstract
Uremic pruritus is a common problem in patients with end-stage renal disease (ESRD), but the underlying mechanisms are not yet fully understood. We aimed to investigate the association between severity of uremic pruritus and cutaneous serine protease activity, as well as proteinase-activated receptor-2 (PAR-2) expression. Twelve ESRD patients with pruritus, 4 ESRD patients without pruritus, and 6 healthy controls were enrolled. Skin biopsies were obtained from the abdomen. Protease activity and PAR-2 expression in the epidermis were examined by in situ zymography and confocal laser microscopy, respectively. All ESRD patients presented more pronounced cutaneous protease activity compared with that in healthy controls. The skin samples from the patients with pruritus showed higher protease activity than either nonpruritic ESRD patients or healthy controls. The epidermis in all samples of ESRD patients presented higher immunoreactivity against PAR-2 versus those of healthy controls. In addition, correlation analysis between PAR-2 expression and VAS pruritus scores showed a significant positive correlation. Our data suggests that levels of serine protease and PAR-2 expression could play important roles in the pathogenesis of uremic pruritus.
Uremic pruritus is a common and burdensome problem affecting up to 86% of patients with end-stage renal disease (ESRD)1,2,3). Although the underlying mechanisms are not yet fully understood, many causative factors have been suggested1,2,3,4,5). These include uremic xerosis, secondary hyperparathyroidism, calcium phosphate crystals, uremic neuropathy, and iron-deficiency anemia4). In addition, immune system abnormalities or changes in the opioidergic system have been proposed to explain the pathophysiology of uremic pruritus5). Histamine, which is a common mediator of various cutaneous disorders associated with an itching sensation, also plays a pathogenic role in some patients with uremic pruritus. However, because many of these patients are refractory to treatment with antihistamines, there must be additional histamine-independent mediators that need to be identified.
Proteinase-activated receptors (PARs) are G-protein-coupled receptors that are activated by certain proteinases6,7). Among the PARs, PAR-2 is cleaved by trypsin-like serine proteases and is associated with acute inflammation7). In addition, PAR-2 in spinal apparent neurons has been shown to have an important role in hyperalgesia through its effects on the pain pathway7). Furthermore, recent investigations have suggested that PAR-2 is one of the histamine-independent mediators of itching6,8). Steinhoff et al.6) demonstrated that significantly enhanced PAR-2 signaling was found in patients with atopic dermatitis, and intradermal injection of PAR-2 agonists induced enhanced and prolonged scratching response. This suggests a role of PAR-2 in the sensation of pruritus. However, neither the status of epidermal PAR-2 expression nor the relationship between PAR-2 and uremic pruritus in ESRD patients, have been studied. In this study, we investigated cutaneous PAR-2 expression and serine protease activity in ESRD patients with pruritus using confocal laser microscopy and in situ zymography, and these were compared to those of healthy controls and nonpruritic ESRD patients.
There were 12 ESRD patients with pruritus, 4 nonpruritic ESRD patients, and 6 healthy controls who were enrolled in this study. The study was approved by the Institutional Review Board of Yonsei University College of Medicine, and informed consent was obtained from all participants. The ESRD status was defined as an estimated glomerular filtration rate lower than 15mL/min/1.73m2, as calculated by the Modification of Diet in Renal Disease equation. No participants had a prior history of any dermatologic disease other than pruritus. Medications related to pruritus except anti-histamine were discontinued at least two weeks before starting the baseline evaluation. At baseline, visual analog scale (VAS) and pruritus grading score for assessing severity of pruritus were recorded4).
Skin biopsy specimens from all participants were obtained from a similar area of the abdomen without remarkable excoriations or crusts. Serine protease activity in the epidermis of 5-µm-thick frozen sections were examined by in situ zymography using the EnzCheck® Protease assay kit (Invitrogen Inc.; Grand Island, NY, USA) as previously described9).
Epidermal PAR-2 expression in biopsy material was visualized by immunofluorescence study. Briefly, paraffin-embedded samples were cut into 4-µm-thick sections and deparaffinized. The deparaffinized sections were incubated for 2 hours at room temperature with a 1:250 dilution of a rabbit anti-human PAR-2 polyclonal antibody (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA, USA) in blocking buffer (1% w/v BSA and 0.1% w/v coldwater fish gelatin in PBS). The sections were washed with PBS and then incubated for 1 hour at room temperature with a 1:100 dilution of fluorescein isothiocyanate-labeled goat anti-rabbit IgG antibody (Santa Cruz Biotechnology, Inc.) in blocking buffer. Stained tissue sections were washed with PBS and then examined with a C1 Plus confocal laser microscope (Nikon; Tokyo, Japan). Fluorescence intensities were analyzed using Metamorph software version 7.7.1.0 (Molecular Devices Inc.; Sunnyvale, CA, USA).
Statistical analyses were performed by the nonparametric Kruskal-Wallis test for multiple comparisons. Significant differences obtained by Kruskal-Wallis analysis were confirmed by Mann-Whitney U test. The relationship between PAR-2 expression and VAS pruritus scores was assessed by spearman's correlation analysis. All analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA) and p-values of <0.05 were considered to be significant.
Baseline characteristics of the participants are summarized in Table 1. The median age of ESRD patients with pruritus was 66 years (ranges from 41 to 79), and male gender was 75%. The median VAS and pruritus score were 5.25 (ranges from 1 to 10). The median age and gender ratio of ESRD patients without pruritus and healthy control were comparable with ESRD patients with pruritus.
All ESRD patients without pruritus presented more pronounced cutaneous protease activity by computer-based analysis of relative fluorescence intensities, compared with that in healthy controls (Fig. 1, p<0.01). In addition, the skin samples obtained from uremic pruritus patients showed higher protease activity than either nonpruritic ESRD patients or healthy controls (p<0.001).
Regardless of pruritus status, the epidermis in all samples of ESRD patients presented higher immunoreactivity against PAR-2 versus those of healthy controls (Fig. 2). Computer-based fluorescence intensity analysis revealed that significantly higher PAR-2 expression relative to healthy controls were found in samples from nonpruritic (p<0.01) and pruritic ESRD patients (p<0.001). Somewhat higher PAR-2 expression were found in samples from pruritic versus nonpruritic ESRD patients, but the difference was not statistically significant. However, PAR-2 expression in the upper half of epidermal layers of skin were significantly higher in samples from pruritic versus nonpruritic ESRD patients (p<0.001) (Fig. 2D).
Interestingly, correlation analysis between PAR-2 expression and VAS pruritus scores in ESRD patients showed a significant positive correlation (Spearman's rho=0.670; p=0.005) (Fig. 3).
The PAR proteins are G-protein coupled receptors characterized by self-activation following specific proteolytic cleavage of their extracellular domains10). Among the four identified PAR members, PAR-2 is activated by trypsin-like serine proteases; whereas, the others are activated by thrombin11). In skin, PAR-2 is abundantly expressed and is known to be a sensor for endogenous and exogenous proteases, and to play numerous pathophysiological roles11,12). In atopic dermatitis, PAR-2 in the skin is markedly expressed on primary afferent nerve fibers and significantly related to pruritus6). Provocation of an itching sensation by intracutaneous injection of endogenous PAR-2 agonists has suggested that this receptor is one of the histamine-independent pruritic mediators, and that PAR-2 might be a new therapeutic target for treatment of itching in atopic dermatitis6).
Uremic pruritus is a common complication in ESRD patients3). Various treatment modalities, such as phototherapy, i.e., with broad-band ultraviolet B and narrow-band ultraviolet B, and oral medications, including antihistamines, gabapentin, gamma linolenic acid, or kappa-opioid receptor antagonist, have been used for the treatment of uremic pruritus with various treatment outcomes4,13,14). In this study, we have investigated the association between the severity of pruritus in ESRD patients and levels of cutaneous serine protease activity, as well as PAR-2 expression. From the results, we hypothesized that uremic pruritus might be related to PAR-2 expression, as in atopic dermatitis. Our data revealed that epidermal PAR-2 expression was significantly increased in ESRD patients compared to healthy controls. In addition, in situ zymography confirmed that protease activity was upregulated in uremic skin. Pruritic ESRD patients showed significantly higher levels of PAR-2 expressed in the skin, especially in the upper epidermis, than that in ESRD patients without pruritus. Previous studies have demonstrated that ESRD patients presented increased serum trypsin levels and decreased trypsin inhibitory capacity15,16). In addition, the protein-energy wasting, which is commonly found in ESRD patients, is attributable to increased levels of specific proteolytic activities, including activation of caspase-3 and the ubiquitin-proteosome system17). We suggest that the pathological milieu in uremic skin might increase proteolytic activity that results in the increased PAR-2 expression in the skin of ESRD patients, and that this mechanism provokes pruritus in such patients.
There are several limitations in this study. First, various etiologic factors associated with uremic pruritus were not fully investigated. Biochemical parameters such as hemoglobin, serum calcium, phosphorous, ferritin, vitamin D, and intact-parathyroid hormone were not measured. Second, the basic investigation about the pathogenesis of how uremia induces PAR-2 expression was not performed. Inflammatory cytokines that might increase protease activities should have been done. Lastly, the number of patients enrolled in this study were very small. However, this is a pilot study so that further evaluations including basic research for pruritus pathway and large scaled clinical trials will be conducted.
In this report, we have demonstrated that protease activity and PAR-2 expression in the epidermis were significantly increased in uremic pruritus patients compared with nonpruritic ESRD patients and healthy controls. In addition, PAR-2 expression and VAS pruritus scores in ESRD patients showed a significant correlation. Although the exact pathogenesis of uremia-induced cutaneous PAR-2 expression remains to be more fully explained, our data does suggest that serine proteases and PAR-2 in the skin could have an important function in the pathogenesis of uremic pruritus.
ACKNOWLEDGEMENTS
This study was supported by Grant 2008 from the Department of Internal Medicine, Yonsei University College of Medicine.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No.2014R1A1A1002439).
References
1. Mistik S, Utas S, Ferahbas A, Tokgoz B, Unsal G, Sahan H, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol. 2006; 20:672–678. PMID: 16836494.

2. Pisoni RL, Wikström B, Elder SJ, Akizawa T, Asano Y, Keen ML, et al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006; 21:3495–3505. PMID: 16968725.

3. Szepietowski JC, Schwartz RA. Uremic pruritus. Int J Dermatol. 1998; 37:247–353. PMID: 9585892.
4. Chen YC, Chiu WT, Wu MS. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis. 2006; 48:69–76. PMID: 16797388.

5. Patel TS, Freedman BI, Yosipovitch G. An update on pruritus associated with CKD. Am J Kidney Dis. 2007; 50:11–20. PMID: 17591521.

6. Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003; 23:6176–6180. PMID: 12867500.

7. Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001; 7:821–826. PMID: 11433347.

8. Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009; 29:6691–6699. PMID: 19458238.

9. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, et al. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008; 128:1930–1939. PMID: 18305573.

10. Déry O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998; 274:C1429–C1452. PMID: 9696685.

11. Nystedt S, Ramakrishnan V, Sundelin J. The proteinaseactivated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996; 271:14910–14915. PMID: 8663011.
12. Rattenholl A, Steinhoff M. Proteinase-activated receptor-2 in the skin: receptor expression, activation and function during health and disease. Drug News Perspect. 2008; 21:369–381. PMID: 19259550.

13. Manenti L, Vaglio A, Borgatti PP. Gabapentin as a therapeutic option in uremic pruritus. Kidney Int. 2008; 73:512. PMID: 18235531.

14. Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005; 16:3742–3747. PMID: 16251241.
15. Montalto G, Lorello D, Carroccio A, Sparacino V, Li Vecchi M, Soresi M, et al. Serum trypsin in chronic renal failure and transplant patients. Am J Gastroenterol. 1992; 87:1175–1179. PMID: 1381554.
16. Hashemi M, Mehrabifar H, Homayooni F, Naderi M, Montazerifar F, Ghavami S. Serum trypsin inhibitory capacity in hemodialysis patients. Saudi J Kidney Dis Transpl. 2009; 20:604–607. PMID: 19587500.
17. Mitch WE. Proteolytic mechanisms, not malnutrition, cause loss of muscle mass in kidney failure. J Ren Nutr. 2006; 16:208–211. PMID: 16825021.

Fig. 1
Computer-based fluorescence intensity analyses. Comparison of serine protease activity was done in healthy controls (n=6), nonpruritic ESRD patients (n=4), and uremic pruritus patients (n=12). *p<0.01, **p< 0.001.
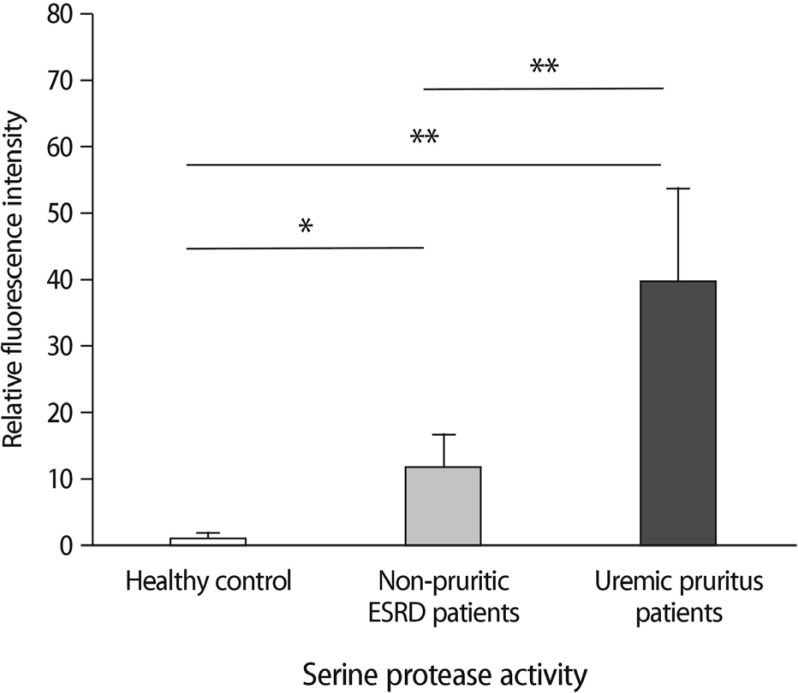
Fig. 2
Confocal laser microscopic study of epidermal PAR-2 expression. The skin samples obtained from (A) healthy controls, (B) nonpruritic ESRD patients, and (C) uremic pruritus patients indicate there was higher PAR-2 expression in uremic pruritus patients than in healthy controls or nonpruritic ESRD patients (original magnification ¡¿400, Bars=100 µm). (D) Computer-based relative fluorescence intensity analyses of PAR-2 expression in the entire epidermis and the upper half of epidermis were done in healthy controls (n=6), nonpruritic ESRD patients (n=4), and uremic pruritus patients (n=12). *p<0.01, **p<0.001.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download