Abstract
Potassium is abundant in the ICF compartment in the body and its excretion primarily depends on renal (about 90%), and to a lesser extent (about 10%) on colonic excretion. Total body potassium approximated to 50mmol/kg body weight and 2% of total body potassium is in the ECF compartment and 98% of it in the intracellular compartment.Dyskalemia is a frequent electrolyte imbalance observed among the maintenance hemodialysis patients. In case of hyperkalemia, it is frequently "a silent and a potential life threatening electrolyte imbalance" among patients with ESRD under maintenance hemodialysis. The prevalence of hyperkalemia in maintenance HD patients was reported to be about 8.7-10%. Mortality related to the hyperkalemia has been shown to be about 3.1/1,000 patient-years and about 24% of patients with HD required emergency hemodialysis due to severe hyperkalemia. In contrast to the hyperkalemia, much less attention has been paid to the hypokalemia in hemodialysis patients because of the low prevalence under maintenance hemodialysis patients. Severe hypokalemia in the hemodialysis patients usually was resulted from low potassium intake (malnutrition), chronic diarrhea, mineralocorticoid use, and imprudent use of K-exchange resins. Recently, the numbers of the new patients with advanced chronic kidney disease undergoing maintenance hemodialysis are tremendously increasing worldwide. However, the life expectancy of these patients is still much lower than that of the general population. The causes of excess mortality in these patients seem to various, but dyskalemia is a common cause among the patients with ESRD undergoing hemodialysis.
The kidney plays a key role in maintaining potassium ([K]) homeostasis by excreting excess potassium. Potassium excretion primarily depends on renal (about 90%), and to a lesser extent (about 10%) on colonic excretion1). However, non-renal excretion of [K] and dialytic [K] removal are important in regulating potassium balance in ESRD patients on hemodialysis because of markedly decreased renal excretion of potassium. Total body potassium is approximately 50mmol/kg body weight and 2% of total body potassium is in the extracellular fluid (ECF) compartment and 98% of it in the intracellular fluid (ICF) compartment2). Oral [K] intake is initially absorbed in the intestine and enters portal circulation. And then, increased ECF[K] stimulates insulin release and in turn, insulin facilitates [K] entry into intracellular compartment by stimulating cell membraneNa+-K+ ATPase3). If it is not for the rapid shift of [K] from the ECF to ICF compartments, serum [K] increased acutely. Excretion of an oral [K] load in the kidney and colon is a relatively slow process, requiring 6-12 hours to be completed. So without rapid transcelluar shift of serum [K] in the human body, we are exposed to hyperkalemic milieu for a while1).
In cases of ESRD patient on maintenance hemodialysis, hyperkalemia seems to be primarily related to poor dietary compliance such as too much [K] intake, inadequate dialysis due to noncompliance or vascular access problems, medications such as ACEIs, [K] sparing diuretics, non-selective beta blockers, NSAIDs, and unfractionate heparin use4). The prevalence of hyperkalemia in any given month of HD patients was reported to be about 8.7-10% depending on individual centers5). Mortality related to the hyperkalemia has been shown to be about 3.1/1,000 patient-years and mainly related to cardiac rhythm disturbances. So, it is frequently called "a silent and a potential life threatening killer" among patients with ESRD under maintenance hemodialysis6). In contrast to hyperkalemia, much less attention has been paid to the hypokalemia in hemodialysis patients because of the low prevalences under maintenance hemodialysis patients. Hypokalemia increases some risks of ventricular arrhythmias in patients with underlying cardiac diseases and a higher incidence of ventricular arrhythmias was reported to increase from 9 to 40% during HD in some studies7). Recently, the numbers of the new patient undergoing maintenance hemodialysis are tremendously increasing worldwide. The cause of excess mortality in these patients seems to bevarious, but dyskalemia is a common cause among the patients with ESRD undergoing hemodialysis. In this article, we are going to review [K] homeostasis in ESRD and how dyskalemia influences morbidity and mortality in maintenance hemodialysis patients.
Potassium plays various roles in the body maintenance of the resting membrane potential and neuromuscular functioning, intracellular acid-base balances, water balances, maintenance of cell volume, cell growth, DNA and protein synthesis, and enzymatic functions8). Daily [K] intake is estimated to range between 50-100mmol, of which 90% of [K] intake is excreted by the kidney and the remainder by the colon. Complete excretion of ingested [K] can be excreted by the kidney in a 6-12 hour period1). Therefore short-term maintenance of ECF [K] concentration depends on extra-renal mechanisms that can respond within a minutes. The majority of total body [K] is located in the intracellular compartment. Many factors influence the distribution of [K] in the body. The factors stimulating [K] shifts from the ECF to ICF compartments include insulin release, cathecolamines, metabolic alkalosis, and anabolic state. Reverse processes happen in mineral acidosis, hyperosmolarity, non-selective beta-blockade use, and alpha-1 stimulation. Potassium is freely filtered at the glomerulus and approximately 65% of filtered load is reabsorbed in the proximal tubule. The collecting duct is the main site of the [K] secretion into the urine8). Factors affecting renal potassium excretion include; distal nephron sodium delivery, the renin-angiotensin-aldosterone system activation, vasopressin status, dietary potassium intake, acid-base status, distal nephron urine flow, and serum potassium concentration. Potassium secretion into the lumen of the distal nephron is passive and this passive movementof potassium is dependent on the concentration gradient across the luminal membrane, the lumen negative electrical gradient (primarily generated by sodium reabsorption) favoring secretion, and the luminal membrane's permeability to potassium9).
Aldosterone plays a key role in the regulation of potassium homeostasis. Aldosterone binds to the nuclear mineralocorticoid receptor within the distal tubule and the principal cells in the cortical collecting duct. At a cellular level, aldosterone opens apical [Na] channels and enhances Na+-K+ ATPase activity on the baso-lateral membranes, resulting in an increase in [K] secretion10). The major stimuli for aldosterone secretion are angiotensinII and elevations in serum [K] level11). Aldosterone also influences extra-renal regulation of [K] secretion via increases in colonic and salivary secretion of [K]12).
Hyperkalemia is defined as a serum [K] concentration-greater than 5.0mEq/L. The kidneys are primarily responsible for [K] excretion in healthy adults, it is not surprising that patients with ESRD are at a high risk population for developing hyperkalemia. At any given level of kidney function, hyperkalemia is more likely to occur in patients who have concurrent medical conditions such as insulin deficiency or concurrent use of drugs to interfere with [K] secretions such as aldosterone antagonists, angiotensin II converting enzyme inhibitors, and/or angiotensin II receptor antagonists9). Patients with chronic renal failure on maintenance hemodialysis develop variety of adaptations to compensate for the decrease of renal [K] excretion. In this population, extra-renal colonic [K] excretion is a paramount importance in defending against hyperkalemia. This colonic [K] adaptation is mediated by increased colonic secretion, which is 2-3 fold higher in patients on hemodialysis than in patients with normal renal function13,14). The process of [K] adaptation is facilitated by the increase in aldosterone secretion associated with increases in serum [K] concentration. The other system defends against hyperkalemia by regulating distribution of potassium between the intracellular and extracellular compartments. Many factors influence [K] distribution between the ICF and ECF compartments. Insulin and cathecolamines are major factors to regulate Na+-K+-ATPase activities over the short term period15). Insulin have a major role in potassium adaptationin end-stage renal disease (ESRD), enhancing cellular [K] uptake16,17). Prolonged fasting has been associated with hyperkalemiain dialysis patients17). Although bicarbonatealone has little or no effect on cellular [K] uptake inpatients with ESRD18), it appears to facilitate insulin'seffect perhaps by correction of acidemia19). Clinical data regarding the role ofaldosterone in [K] handling in dialysis patients is equivocal20). However, with recent controlled trial fails toshow a convincing [K]-lowering effect of fludrocortisones21). Secondary hyperparathyroidism is a common feature of ESRD patients. It appears to decrease cellular uptake of [K] via an increase of intracellular calcium, which suppress oxidative metabolism and ATP generation of cell and reduce Na+-K+-ATPase activity22). Extracellular hypertonicity, usually seen with diabetic hyperglycemia23) or hypertonic fluid administration24), causes hyperkalemia on the basisof convective [K] efflux from cells and insulin deficiency. Lastly, severe exercise25) and hemolysis in the course of hemodialysis26) release large amount of [K] into the ECF compartment and may cause severe hyperkalemia.
True hyperkalemias on maintenance hemodialysis occur by increased dietary [K] intake in interdialytic interval, absence of residual renal function which is the main sources of [K] removal in the normal persons, reduction in dialysis [K] clearance, hypoaldosteronism, metabolic acidosis usually seen in ESRD, hypercatabolic state, blood transfusions, abnormal colonic [K] secretion, and various drugs used in hemodialysis patients (e.g. cox 1&2 inhibitors, beta blockers, ACE inhibitors, Angiotensin Receptor blockers, postassium sparing diuretics, succinylcholine, digoxin, cyclosporine, tacrolimus, ketoconazol, potassium containing drugs)3,9,20,24,27). Chronic renal failure is the most common cause of [K] retention, especially when GFR falls toward 20% of normal. In most patients with nonoliguric chronic renal failure, mild hyperkalemia is usual28). In cases of chronic renal failure due to diabetes mellitus and tubulointerstitial diseases, hyperkalemia is more pronounced because of low circulating renin and aldosterone levels29). Many drugs used in the intensive care unit (ICU) can produce hyperkalemia by decreasing [K] excretion30). Shift of the intracellular [K] to extracellular compartment may lead to severe hyperkalemia in critically ill patients. The traditional concept wasthat metabolic acidosishas been implicated as a causative factor of hyperkalemia31). This paradigm has been disproved, and changes in serum [K] in relation to acid-base disorders are more complex than initially thought. The most common forms of acute metabolic acidosis in critically ill patients, diabetic ketoacidosis and lactic acidosis, are not associated with shift [K] out of cells32). Hyperkalemia seen with diabetic ketoacidosis is most likely caused by increased release of intracellular [K] due to the breakdown of muscle cells. Hypertonicity of the extracellular fluid causes water to exit cells, and [K] is swept out along with water. Unless renal function is not adequate to eliminate the excess [K], hyperkalemia occurs. This clinical setting may occur in patients with uncontrolled diabetes and renal insufficiency and can lead to severe hyperkalemia33). Massive tissue breakdown such as trauma, burn, and rhabdomyolysis, can lead to release of [K] into the extracellular space. If renal functions are severely impaired, hyperkalemia may develop. Drugs that impair [K] entry into cells can affect the transmembrane balance of [K]. β-Adrenergic blockers inhibit the entry of [K] into cells and, in cases with renal failure, can accelerate development of hyperkalemia34). Succinylcholine blocks normal reentry of [K] into cells after depolarization and causes a temporary increase in serum [K]35). Digoxin, an inhibitor of cell membrane Na+-K+-ATPase, impairs [K] entry into cells, but does not cause significant hyperkalemia at usual therapeutic dose36). However, it sometimes may cause hyperkalemia with toxic doses37).
Clinical symptoms related to hyperkalemia are nonspecific and most often, asymptomatic. However, it affects cardiac and neuromuscular cells which are particularly sensitive to changes in serum [K]. In patients with mild to moderate hyperkalemia, patients usually complain of muscle weakness, fatigue, paresthesias, palpitations, and cardiac arrythmias. As evidenced by characteristic changes in the electrocardiogram(ECG) that serve as indicators of potential life-threatening arrhythmias, the first sign of increased serum [K] is tenting of the T wave. ECG changes may progress rapidly in conjunction with serum [K] levels. ECG changes include widening of the QRS complex, progressive development of atrioventricular conduction blocks, slow idioventricular rhythm, an ECG tracing that looks like a sine wave, ventricular fibrillation, and finally asystole38). ECG findings are not always sensitive to changes in serum [K] levels and there is no absolute level of serum [K] associated with a particular ECG abnormality. Sometimes, normal ECG findings have been described with severe hyperkalemia, and in some cases the first manifestation of cardiac changes from hyperkalemia may be ventricular fibrillation39,40). Hyperkalemia can cause neuromuscular symptoms such as paresthesias and weakness in the arms and legs, followed by a symmetrical flaccid paralysis of the extremities that ascends toward the trunk, finally involving the respiratory muscles. The cranial nerves are usually not affected by hyperkalemia1).
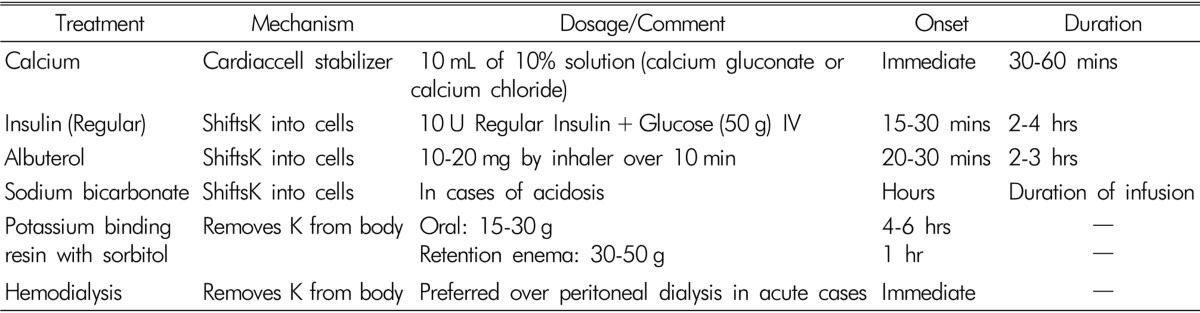
Regardless of the cause, the primary goal of treating hyperkalemia in HD patients is to prevent adverse cardiac arrythmias. Therapy to lower serum [K] should be started immediately when serum [K] level is greater than 6.5mEq/L or if the ECG changes show sings of conduction abnormalities. Treatment modalities are aimed at one of three mechanisms to prevent or decrease these complications: (1) direct antagonism of hyperkalemic effect on the cell membrane polarization, (2) movement of extracellular [K] into the intracellular compartment, and (3) removal of [K] from the body. Table 1 summarizes acute management of hyperkalemia41). However, in patients with hemodialysis, residual renal function is not sufficient to promote kaliuresis.If hyperkalemia is not urgent, sodium polystyrene sulfonate (Kayexalate) and calcium polystyrene sulfonate (Kalimate) can be used. At equilibrium, each gram of resin removes about 0.5 to 1mEq of [K]. The usual dose of [K]-binding resins are 15 to 30 g orally. When given by mouth, [K]-binding resin has little effect for 4-6 hours because it must transit to the colon to be effective. When given as a retention enemas consisting of 30 to 50 g of the resin in 70% sorbitol solution, it works within 1 hour. It is important, however, that the enema should be retained for at least 30 to 60 minutes to obtain the desired therapeutic effect. Unfortunately, the combined use of sorbitol and Kayexalate can cause bowel necrosis and perforation. These complications seem to be more likely in severely immunocompromised patients or shortly after surgery42). In acute cases when serum [K] needs to be corrected rapidly, hemodialysis is a preferred mode of therapy. Hemodialysis can quickly remove 70 to 150mEq of [K] and should be used asa gold standardtreatment modality when other treatments fail43). In addition to the implementation of rapid treatment, the causes of hyperkalemia should be sought and immediatedlycorrected, and offending drugs should be discontinued when possible. In cases of chronic hyperkalemia, dietary restriction of [K] is the mainstay of management in these patients (40-70mEq/day). If acidosis is present, sodium bicarbonate is helpful by increasing distal nephron sodium delivery, inducing kaliuresis, and promoting intracellular potassium shift. An additional alternative is the use of [K]-binding resins such as Kayexalate or Kalimate combined with sorbitol to avoid constipation, at smaller doses given daily or every other day.
Hypokalemia is usually defined as serum [K] less than 3.5mEq/L. Hypokalemia usually occurs as a consequence of [K] depletion due to either increased excretion or inadequate intake. However, shift of [K] in the extracellular to intracellular compartmentsalso can cause hypokalemia. In cases of ESRD patients on hemodialysis, hypokalemia is a relatively rare event comparing to hyperkalemia. The precise prevalence of hypokalemia in maintenance HD patients is unknown but the prevalence is various among different centers5,44,45). Most hypokalemic patients are asymptomatic depending on serum [K] levels but it can be associated with mild muscle weakness to serious sudden cardiac death. The consequences of hypokalemia result to alterations in the resting membrane potential of cardiac and neuromuscular cells. The most serious and potentially fatal effects of hypokalemia are related to disturbances in cardiac rhythm that can lead to cardiac arrest. However, cardiac arrest caused by hypokalemia occurs almost exclusively in patients with underlying cardiac disease or patients taking digitalis. Characteristic electrocardiographic (ECG) changes associated with hypokalemia include broad, flat T waves, ST depression, the appearance of U waves, QT interval prolongation, and finally ventricular arrhythmias leading to cardiac arrest46). When serum [K] is less than 3.0mEq/L, generalized weakness can develop and serum [K] decreases to less than 2.5mEq/L, muscle necrosis and rhabdomyolysis can occur. With progression of hypokalemia, an ascending muscle paralysis develops, leading to respiratory failure and arrest5,8). Hypokalemia in maintenance hemodialysis patients is less frequent condition compared to hyperkalemia (0.3-0.5% Vs. 8.7-10%)5) and can be caused by low dietary potassium intake, malnutrition, chronic diarrhea, prescription of drugs that can increase colonic [K] excretions such as mineralocorticoids and imprudent use of [K]-exchange resins8,45). One recent study conducted on Non Dialysis Dependent (NDD)-CKD population, overall mortality was significantly associated with both higher and lower serum potassium levels even after adjustments for relevant confounders. They also found that hypokalemias were significantly associated with faster loss of kidney function over time, even after adjusting for other known risk factors such as BP, proteinuria and comorbid conditions. They concluded that hypoand hyper-kalemia are associated with higher mortality in NDD-CKD patients47).
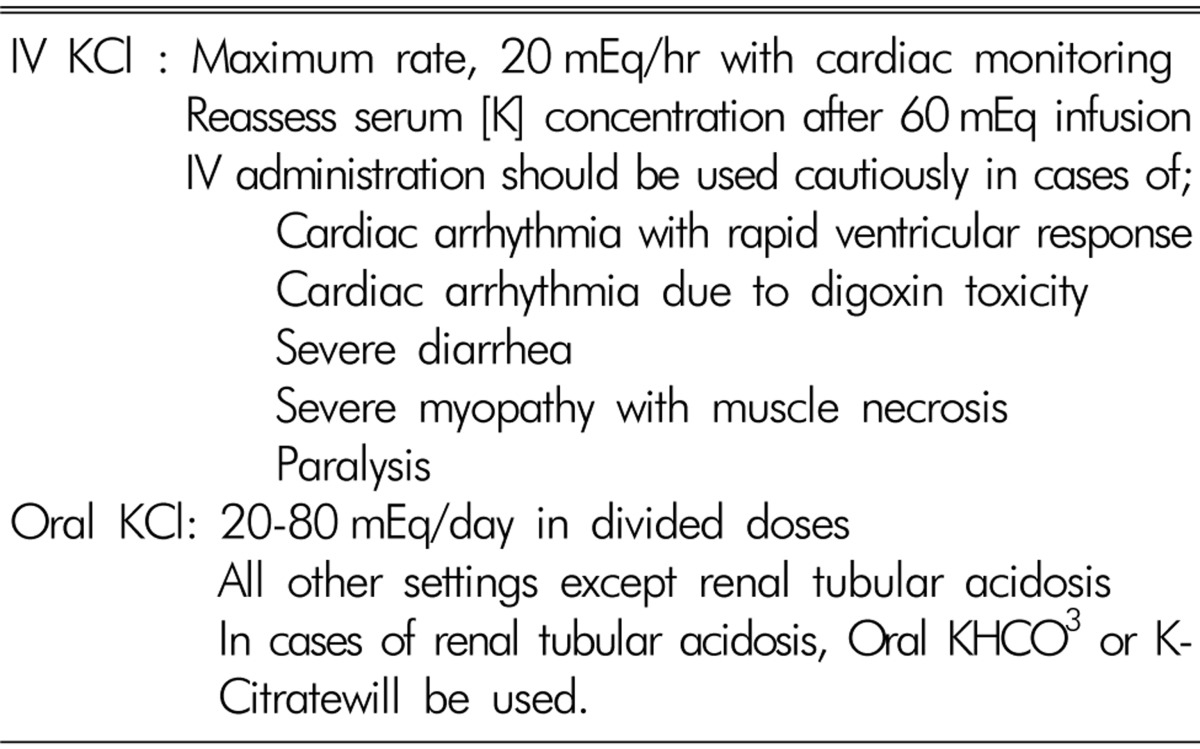
The goal of treatment in hypokalemia on maintenance hemodialysis is to prevent cardiac rhythm disturbances and serious neuromuscular weaknesses. Supplementation of [K] is the main treatment for hypokalemia and is usually achieved with the oral administration of potassium preparations. There are four types of potassium preparations: potassium chloride, potassium phosphate, potassium bicarbonate and potassium citrate. Potassium phosphate is used to treat hypokalemia with hypophosphatemia. Potassium bicarbonate or citrate is preferred in patients with hypokalemia and metabolic acidosis. In all other settings, potassium chloride should be used. Conditions requiring emergent therapy are usually rare. In general, plasma [K] decreases by approximately 0.3mEq/L for each 100mEq decrease in total body [K]. Potassium replacement should be given orally except when severe hypokalemia is associated with respiratory or cardiac instability, in which case the IV route is prefered. When given intravenously, the rate of [K] administration should not exceed 20mmol/hourto minimize possible iatrogenic hyperkalemia. For IV infusion of [K], an infusion pump and continuous cardiac monitoring are mandatory. When potassium is administered intravenously through a peripheral vein, the concentrations should not exceed 50mmol/l. IV fluids containing higher [K] concentration are often painful and cause peripheral vein irritation. Serum [K] level should be followed closely, especially when using IV route or higher doses, to prevent the development of hyperkalemia because ESRD patients with MHD have no residual renal function to excrete excess potassium48). Table 2 shows summaries of the treatment of hypokalemia3).
Dyskalemia is a frequent electrolyte imbalance observed among the maintenance hemodialysis patients. In case of hyperkalemia, it is frequently "a silent and a potential life threatening electrolyte imbalance" among patients with ESRD under maintenance hemodialysis. The prevalence of hyperkalemia in HD patients was reported to be about 8.7-10%. Mortality related to hyperkalemia has been shown to be about 2-5% of deaths among patients with ESRD5) and about 24% of patients with HD required emergency hemodialysis due to severe hyperkalemia1). In contrast to the hyperkalemia, hypokalemia in maintenance hemodialysis patients is less frequent condition. The precise prevalence of hypokalemia in maintenance HD patients is unknown but the prevalence is various among different centers5,44,45). Most hypokalemic patients are asymptomatic depending on serum [K] levels but it can be associated with mild muscle weakness to serious sudden cardiac death. It can be caused by low dietary potassium intake, malnutrition, chronic diarrhea, prescription of drugs that can increase colonic [K] excretions such as mineralocorticoids and [K]-exchange resins8,45). Much less attention has been paid to the hypokalemia in hemodialysis patients because of the low prevalence under maintenance hemodialysis patients. However, in cases of severe hypokalemia, we also pay attention to prevent cardiac rhythm disturbances and serious neuromuscular weaknesses.
References
1. Putcha N, Allon M. Management of Hyperkalemia in Dialysis Patients. Semin Dial. 2007; 20:431–439. PMID: 17897250.

2. Greenberg A. Hyperkalemia: Treatment options. Semin Nephrol. 1998; 18:46–57. PMID: 9459288.
3. Gennari FJ. Disorders of potassium homeostasis: Hypokalemia and Hyperkalemia. Crit Care Clin. 2002; 18:273–288. PMID: 12053834.
4. Khedr E, Abdelwhab S, El-Sharkay M, Ali M, El Said K. Prevalence of hyperkalemia among hemodialysis patients in Egypt. Renal Failure. 2009; 31:891–898. PMID: 20030523.

5. Tzamaloukas AH, Avasthi PS. Temporal profile of serum potassium concentration in nondiabetic and diabetic outpatients on chronic dialysis. Am J Nephrol. 1987; 7:101–109. PMID: 3605230.

6. Weiner ID, Wingo CS. Hyperkalemia: A. Potential Silent Killer. J Am Soc Nephrol. 1998; 9:1535–1543. PMID: 9697678.

7. Abe S, Yoshizawa M, Nakanishi N, et al. Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J. 1996; 131:1137–1144. PMID: 8644592.

8. Hoskote SS, Joshi SR, Ghosh AK. Disorders of potassium homeostasis: Pathophysiology and management. JAPI. 2008; 56:685–725. PMID: 19086355.
9. Evans KJ, Greenberg A. Hyperkalemia: A Review. J Intensive Care Med. 2005; 20:272–290. PMID: 16145218.

10. Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int. 2000; 57:1324–1328. PMID: 10760062.

11. Young DB, Smith MJ Jr, Jackson TE, Scott RE. Multiplicative interaction between angiotensin II and K concentration in stimulation of aldosterone. Am J Physiol. 1984; 247:E328–E345. PMID: 6476112.

12. Bastl CP, Hayslett JP. The cellular action of aldosterone in target epithelia. Kidney Int. 1992; 42:250. PMID: 1405311.

13. Martin RS, Panese S, Virginillo M, et al. Increased secretion of potassium in the rectum of man with chronic renal failure. Am J Kidney Dis. 1986; 8:105–110. PMID: 3740056.
14. Sandle GI, Tapster S, Goodship TH. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci (Lond). 1987; 73:247–252. PMID: 3652631.

15. Clausen T, Everts ME. Regulation of the Na-K-pump in skeletal muscle. Kidney Int. 1989; 35:1–13. PMID: 2540370.
16. Allon M, Dansby L, Shanklin N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am J Med. 1993; 94:475–482. PMID: 8498392.

17. Allon M, Takeshian A, Sanklin N. Effect of insulin-plus-glucose infusion with or without epinephrine on fasting hyperkalemia. Kidney Int. 1993; 43:212–217. PMID: 8433561.

18. Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992; 41:369–374. PMID: 1552710.

19. Kim HJ. Combined effect of bicarbonate and insulin with glucose in acute therapy of hyperkalemia in end-stage renal disease patients. Nephron. 1996; 72:476–482. PMID: 8852501.

20. Ahmed J, Weisberg LS. Hyperkalemia in dialysis patients. Semin Dial. 2001; 14:348–356. PMID: 11679104.

21. Kaisar MO, Wiggins KJ, Sturtevant JM, et al. A randomized controlled trial of fludrocortisone for the treatment of hyperkalemia in hemodialysis patients. Am J Kidney Dis. 2006; 47:809–814. PMID: 16632019.

22. Massry SG. Renal failure, parathyroid hormone and extrarenal disposal of potassium. Miner Electrolyte Metab. 1990; 16:77–81. PMID: 2182997.
23. Goldfarb S, Cox M, Singer I, Goldberg M. Acute hyperkalemia induced by hyperglycemia: hormonal mechanisms. Ann Intern Med. 1976; 84:426–432. PMID: 769633.

24. Moreno M, Murphy C, Goldsmith C. Increase in serum potassium resulting from the administration of hypertonic mannitol and other solutions. J Lab Clin Med. 1969; 73:291–298. PMID: 5764025.
25. Clark BA, Shannon C, Brown RS, Gervino EV. Extrarenal potassium homeostasis with maximal exercise in end-stage renal disease. J Am Soc Nephrol. 1996; 7:1223–1227. PMID: 8866416.

26. Sweet SJ, McCarthy S, Steingart R, Callahan T. Hemolytic reactions mechanically induced by kinked hemodialysis lines. Am J Kidney Dis. 1996; 27:262–266. PMID: 8659503.

27. Kim HJ. Pathogenesis and treatment of dyskalemia in maintenance hemodialysis and CAPD. Electrolyte Blood Press. 2006; 4:47–52.

28. Kupin WL, Narins RG. The hyperkalemia of renal failure: Pathophysiology, diagnosis and therapy. Contrib Nephrol. 1993; 102:1–22. PMID: 8416175.

29. DeFronzo RA. Hyperkalemia and hyporeninemic hypoaldosteronism. Kidney Int. 1980; 17:118–134. PMID: 6990088.

30. Buckley MS, Leblanc JM, Cawley MJ. Electrolyte disturbances associated with commonly prescribed medications in the intensive care unit. Crit Care Med. 2010; 38:S253–S264. PMID: 20502178.

31. Burnell JM, Scribner BH, Uyeno BT, Villamil MF. The effect in humans of extracellular pH change on the relationship between serum potassium concentration and intracellular potassium. J Clin Invest. 1956; 35:935–939. PMID: 13367188.

32. Adrogue HJ, Madias NE. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981; 71:456–467. PMID: 7025622.
33. Goldfarb S, Cox M, Singer I, Goldberg M. Acute hyperkalemia induced by hyperglycemia: Hormonal mechanisms. Ann Intern Med. 1976; 84:426–432. PMID: 769633.

34. Arrizabalaga P, Montoliu J, Martinez Vea A. Increase in serum potassium caused by beta-2 adrenergic blockade in terminal renal failure: Absence of mediation by insulin or aldosterone. Proc Eur Dial Transplant Assoc. 1983; 20:572–576. PMID: 6318223.
35. Gronert GA. Succinylcholine-induced hyperkalemia and beyond.1975. Anesthesiology. 2009; 111:1372–1377. PMID: 19934884.
37. Rees SM, Nelson LN. Digoxin, hyperkalemia, and kidney failure. Ann Emerg Med. 1997; 29:694–695. PMID: 9140260.
38. Parham WA, Mehdirad AA, Biermann KM, Freman CS. Hyperkalemia Revisited. Tex Heart Inst J. 2006; 33:40–47. PMID: 16572868.
39. Szerlip HM, Weiss J, Singer I. Profound hyperkalemia without electrocardiographic manifestations. Am J Kidney Dis. 1986; 7:461–465. PMID: 3717152.

40. Dodge HT, Grant RP, Seavey PW. The effect of induced hyperkalemia on the normal and abnormal electrocardiogram. Am Heart J. 1953; 45:725–740. PMID: 13040269.

41. Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008; 36:3246–3251. PMID: 18936701.

42. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-Exchange Resins for the Treatment of Hyperkalemia: Are they Safe and Effective? J Am Soc Nephrol. 2010; 21:733–735. PMID: 20167700.

43. Musso CG. Potassium metabolism in patients with chronic kidney disease. Part II: Patients on dialysis (stage 5). Int Urol Nephrol. 2004; 36:469–472. PMID: 15783126.

44. Hwang SH, Kim HJ. Distribution of serum potassium concentration and analysis of associated factors with hyperkalemia in chronic hemodialysis patients. Korean J Med. 1996; 50(1):87–93.
45. Hwang J-C, Wang C-T, Chen C-A, Chen H-C. Hypokalemia is associated with increased mortality rate in chronic hemodialysis patients. Blood Purif. 2011; 32:254–261. PMID: 21849775.

47. Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012; 120:c8–c16. PMID: 22156587.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download