Abstract
Catecholamine secretory traits were significantly heritable, as were stress-induced blood pressure changes. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in catecholamine biosynthesis. In the tyrosine hyroxylase promoter, significant associations were found for urinary catecholamine excretion and for blood pressure response to stress. TH promoter haplotype 2 (TGGG) showed pleiotropy, increasing both norepinephrine excretion and blood pressure during stress. In hypertension, 2 independent case-control studies (1,266 subjects with 53% women and 927 subjects with 24% women) replicated the effect of C-824T in the determination of blood pressure. Chromogranin A (CHGA) plays a fundamental role in the biogenesis of catecholamine secretory granules. Changes in the storage and release of CHGA in clinical and experimental hypertension prompted us to study whether genetic variation at the CHGA locus might contribute to alterations in autonomic function, and hence hypertension and its target organ consequences such as hypertensive kidney disease (nephrosclerosis). Systematic polymorphism discovery across the human CHGA locus revealed such regulatory regions as the proximal promoter and 3'-UTR. In chromaffin cell-transfected CHGA 3'-UTR and promoter/luciferase reporter plasmids, the functional consequences of the regulatory/non-coding allelic variants were documented. Variants in both the proximal promoter and the 3'-UTR displayed statistical associations with hypertension and hypertensive end stage renal disease. Therefore, I would like to review the common genetic variation in TH and CHGA as a cause of inter-individual variation in sympathetic activity, and ultimately blood pressure and hypertensive kidney disease.
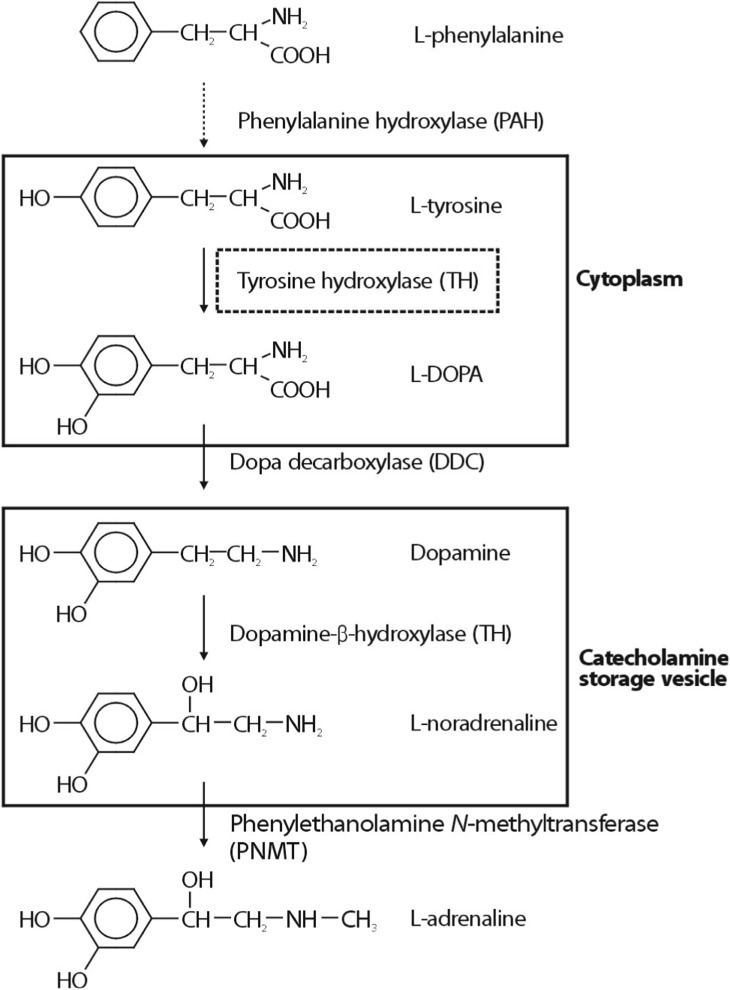
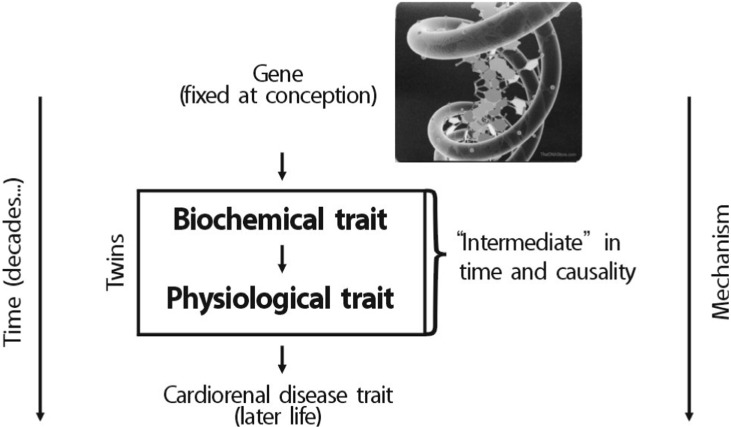
Chromogranin A (CHGA) is the major protein co-stored and co-released with catecholamines from secretory vesicles in adrenal medulla and postganglionic sympathetic axons1). CHGA is required for the formation of catecholamine secretory vesicles in chromaffin cells2). CHGA is also a pro-hormone that gives rise to biologically active peptides such as the dysglycemic peptide pancreastatin3), the antimicrobial peptide chromacin4), the vasodilator vasostatin, and catestatin that acts to inhibit catecholamine release5,6). Over the past 20 years, phenotypic links between CHGA and hereditary (essential, idiopathic, and genetic) human7,8,9) hypertension have been repeatedly observed. Increased serum CHGA has been detected not only in patients with essential hypertension8) but also hypertensive consequences such as cardiac or renal failure7). Researchers observed parallel findings on CHGA secretion and autonomic stress responses in humans10,11), suggesting that an optimal amount of CHGA may be required to establish appropriate catecholamine storage and release, and hence BP homeostasis. I will concentrate on polymorphism discovery at specific points in the catecholamine biosynthetic pathway (Fig. 1), especially tyrosine hydroxylase (TH), as outlined below, and link polymorphism to rigorously defined human phenotypes, both "intermediate" phenotypes in twin pairs (Fig. 2) and ultimate disease phenotypes (hypertension and hypertensive kidney disease).
A common haplotype of the CHGA proximal promoter region, CGATA(at T-1014C, T-988G, G-462A, C-415T, and A-89C), blunted the BP response to cold stress, and the response exhibited molecular heterosis (most extreme phenotype in heterozygotes) between the two most common promoter haplotypes (CGATA/TTGTC)12). Homozygosity for the minor alleles at T-1014C (C/C), T-988G (G/G), and G-462A (A/A) predicted lower stress BP increments with peak prediction for G-462A (rs9658634) heterozygotes (6-7mmHg). G-462A also predicted resting BP in the population, accounting for 3/2mmHg SBP/DBP, once again with the appearance of molecular heterosis (i.e., highest BP in G/A heterozygotes)12). In transfected CHGA promoter/luciferase reporters, CGATA had diminished expression compared to TTGTC, under both basal conditions and after secretory activation by pre-ganglionic stimuli (nicotinic cholinergic, vasoactive intestinal polypeptide). Findings of molecular heterosis were also demonstrated for the transfected CHGA promoter in cella, wherein the diploid combination of the two alleles at G-462A (G/A heterozygosity) gave rise to greater luciferase expression than either allele in isolation12).
A common (27% allele frequency) genetic variant in the CHGA 3'-UTR(C+ 87T; rs7610) was strongly associated with human essential hypertension, accounting for up to 12/9mmHg of BP in men, or 1.9/1.2% of population SBP/DBP variance, especially in males13). The 3'-UTR variant also predicts environmental stress induced increments in blood pressure: the same allele (+87T) that diminished basal BP in the population also decreased the SBP response to stress by 12mmHg (p=0.017), and the response was smaller in women (by 6mmHg, p=0.009), suggesting a mechanism for the early effects of the gene on a pathogenic series of events eventuating in sustained blood pressure elevation13). The 3'-UTR variant is in a region of sequence conservation across species, and acts to change CHGA gene expression in chromaffin cells. In a chromaffin cell-transfected CHGA 3'-UTR/ luciferase reporter plasmid, the +87T allele associated with lower BP also decreased reporter expression by 30% (p=0.009)13).
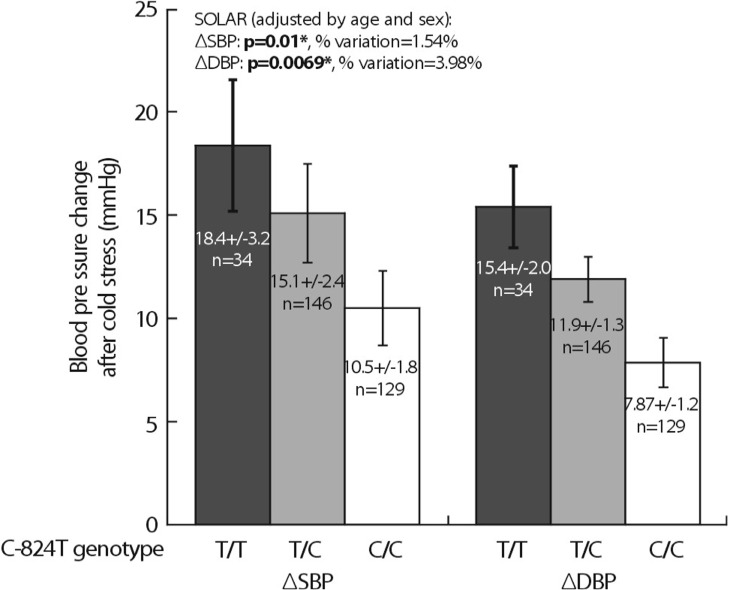
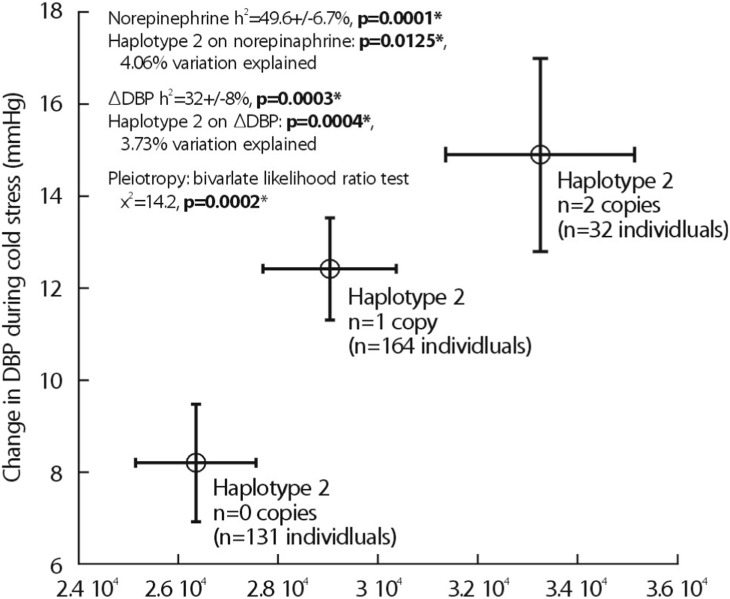
Heritability (abbreviated h2) is the fraction of variance (V=[standard deviation]2) of a phenotype (P) that results from heredity or gene action (h2=VG/VP), as opposed to environmental influences (VE). Heritability is typically reported on a scale of 0 to 1, where h2=1 would represent complete heritability of a trait, and h2=0 would represent complete determination by environment; alternatively, heritability may be expressed on a scale of 0% → 100%, in which case h2=100% would represent complete heritability14,15). The UCSD Twin Project allows to quantify h2 using the classical human twin design. In a study related to this project, 4 common promoter variants in TH were powerful predictors of both biochemical (Fig. 3) and physiological traits (Fig. 4). In particular, common promoter variants C-824T and A-581G were strong predictors of both catecholamine excretion (Fig. 3) and the BP response to environmental stress (Fig. 4). TH promoter haplotypes (e.g., haplotype 2, TGGG) coordinately affected both biochemical traits and physiological stress traits in an illustration illustration of pleiotropy, confirmed by bivariate analysis in SOLAR (Fig. 5).
As an initial approach to genetic effects on the complex trait of hypertension, Ernie Beutler (Scripps Clinic) et al. conducted a relatively unbiased ascertainment of subjects with the most extreme DBPs in a San Diego primary care population; the results were already published in Hypertension16). They illustrate this population-based case/control study, in which >1,200 subjects were drawn from the highest and lowest 5th percentile of blood pressure in a primary care practice of >53,000 adults. In a 2-way ANOVA, there was a significant sex-by-genotype (TH C-824T) interaction on DBP (p=0.044). As expected, sex itself also influenced DBP (p<0.001). When the ANOVA was run in the presence or absence of C-824T, the results indicated that C-824T variation accounted for ~3.4% of population DBP variance. When analyses were separately conducted on the sexes, the C-824T genotype effect was found in males (p=0.045), though not females (p=0.985). Inspection of the bar graph indicated that increasing numbers of the minor (T) allele increased DBP in males, though not females16).
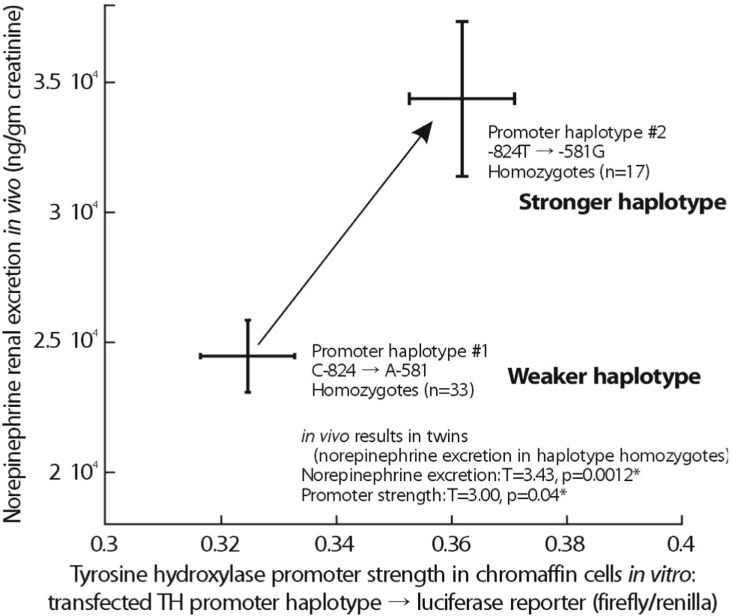
Since phenotypic associations mapped onto the TH promoter region, Daniel T. O'Connor et al. directly tested the functional significance of common variation in the promoter region, by assaying haplotype-specific gene expression in PC12 chromaffin cells, with TH promoter/luciferase reporters (in pGL3-Basic) for the two most common promoter haplotypes: #1 (CGAA, the most common variant) and #2 (TGGG, the second most common variant). Haplotype #2 was substantially more active in chromaffin cells; Fig. 6 illustrates the increased activities of haplotype #2 both in vitro (driving transcription) and in vivo (determining norepinephrine secretion). Relatively low-expressing haplotype #1 (CGAA), is the most common variant in Asian (61.7%), white (46.8%) and Hispanic (37.1%) populations, unusually low(15.4%) in blacks, while higher-expressing haplotype #2 (TGGG), is the most common variant in blacks (at 34.6%).
In African-Americans with a clinical diagnosis of hypertensive kidney disease (or nephrosclerosis)17), genetic variation at CHGA predicts risk for developing the trait, and the peak effect lies in haplotypes spanning the 3'-end of the gene. They then found that CHGA polymorphism predicted the occurrence of hypertensive kidney disease in African-Americans with the peak risk conferred by variation in the 3'-region of the gene17). In this case/control study of hypertensive kidney disease, 3'-UTR variation was associated with ESRD17), while promoter variation resulting in alterations of the nuclear hormone receptor trans-activation may influence blood pressure12).
In subjects from the NIDDK AASK(African-American Study of Kidney Disease and Hypertension) trial, the chronic decline rate of GFR was influenced by genetic variation at CHGA, with the most prominent effect (p=0.006) from haplotype-1 in the 3'-haplotype block, spanning 3'-UTR C+ 87T/rs761018). The effect was also seen for diploid haplotype pairs in that block (p=0.007)18). Finally, mechanistic studies were undertaken initiated by the clue that CHGA promoter variation predicted circulating endothelin-1. In cultured endothelial cells, CHGA triggered co-release of not only the vasoconstrictor and pro-fibrotic endothelin-1, but also the pro-coagulant von Willebrand Factor and the pro-angiogenic angiopoietin-218). These findings, coup led with stimulation of endothelin-1 release from glomerular capillary endothelial cells by CHGA, suggest a plausible mechanism whereby genetic variation at the CHGA locus eventuates in alterations in human kidney function.
Thus, common genetic variation across the human CHGA and TH locus is associated with hypertension and hypertensive kidney disease traits as well as their precursor (or "intermediate") phenotypes. The associated variants are functional when tested in cellular systems. Such findings may lead to novel approaches to the pathophysiology, diagnosis, and treatment of the autonomic dysfunction predisposing to such disease traits.
Acknowledgment
I am very thankful to my mentor, Dr. Daniel T. O'Connor for sharing his own materials for this review.
References
1. Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, O'Connor DT. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990; 81:185–195. PMID: 2404624.

2. Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005; 115:1942–1952. PMID: 16007257.

3. Cadman PE, Rao F, Mahata SK, O'Connor DT. Studies of the dysglycemic peptide, pancreastatin, using a human forearm model. Ann NY Acad Sci. 2002; 971:528–529. PMID: 12438174.

4. Strub JM, Goumon Y, Lugardon K, Capon C, Lopez M, Moniatte M, et al. Antibacterial activity of glycosylated and phosphorylated chromogranin A-derived peptide 173-194 from bovine adrenal medullary chromaffin granules. J Biol Chem. 1996; 271:28533–28540. PMID: 8910482.

5. Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997; 100:1623–1633. PMID: 9294131.

6. Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999; 274:2920–2928. PMID: 9915830.
7. Hsiao RJ, Parmer RJ, Takiyyuddin MA, O'Connor DT. Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore). 1991; 70:33–45. PMID: 1988765.
8. O'Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985; 7:I76–I79. PMID: 3997234.
9. Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O'Connor DT. Chromogranin A. Storage and release in hypertension. Hypertension. 1990; 15:237–246. PMID: 2406199.

10. Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, et al. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens. 2010; 28:817–825. PMID: 20139771.

11. Vaingankar SM, Li Y, Corti A, Biswas N, Gayen J, O'Connor DT, Mahata SK. Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic "rescue" of disease phenotypes in the mouse Chga nockout. Physiol Genomics. 2010; 41:91–101. PMID: 20009010.
12. Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, et al. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int. 2008; 74:115–125. PMID: 18432188.

13. Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, et al. Naturally occurring human genetic variation in the 3'-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sexdependent fashion. J Am Coll Cardiol. 2008; 52:1468–1481. PMID: 19017515.

14. O'Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, Taylor PW, Parmer RJ. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000; 2:16–22. PMID: 10982526.
15. Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006; 47:331–333. PMID: 16446388.
16. Rana BK, Insel PA, Payne S, Abel K, Beutler E, Ziegler MG, Schork NJ, O'Connor DT. Genetic Basis of a Complex Human Trait: Genes Interact with Sex to Determine Blood Pressure in a Very Large Population-Based Sample. Hypertension. 2007; 49:96–106. PMID: 17159089.
17. Salem RM, Cadman PE, Chen Y, Rao F, Wen G, Hamilton BA, et al. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008; 19:600–614. PMID: 18235090.

18. Chen Y, Mahata M, Rao F, Khandrika S, Courel M, Fung MM, et al. Chromogranin A regulates renal function by triggering Weibel-Palade body exocytosis. J Am Soc Nephrol. 2009; 20:1623–1632. PMID: 19520754.

Fig. 3
Influence of tyrosine hydroxylase promoter polymorphism (C-824T) on catecholamine excretion in twins.
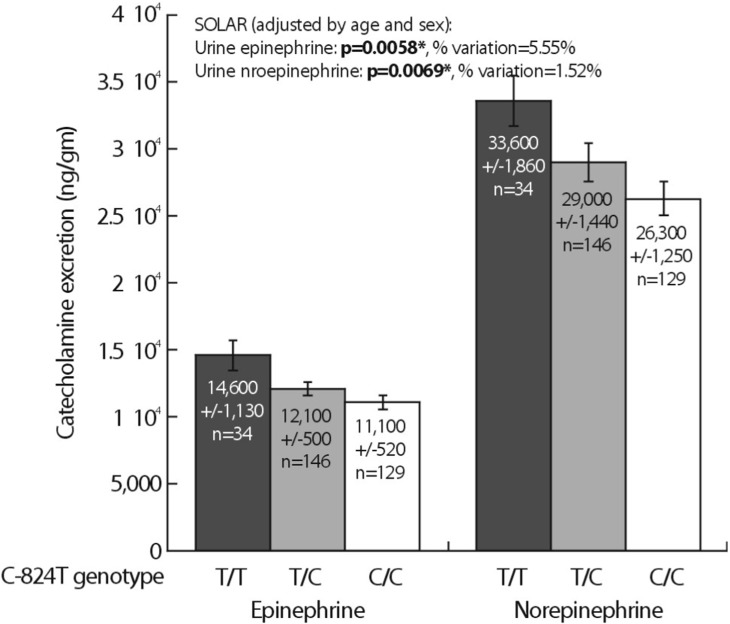
Fig. 4
Influence of tyrosine hydroxylase promoter polymorphism (C-824T) on blood pressure response to stress in twins.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download