Introduction
Since the first invention of atomic forced microscopy (AFM) by Binnig et al., it was recognized as an important imaging tool in biological research1). AFM differs from conventional optical and electron microscopy, because it is performed by sensing the interacting force between its probe tip and sample surface. Furthermore, measuring the force acting between its probe tip and the sample, by means of force-distance curves, is important in defining the sample's physical property. Thus, the AFM force curve can be used to evaluate the stiffness and adhesive properties of cell membranes as the tip moves to and from the sample surface2). Early on, AFM was recognized for its potential to observe living animal cells3). As a result of technical innovations, it is now also possible to perform imaging in any environment including aqueous solutions using AFM.
In renal physiology, AFM is mainly used to image intact renal cells, or to study sub-cellular structures. However, workers interested in renal cells and their membrane transporter function, can now use AFM since it is an ideal method for examining membrane structures. These experiments are still essential and continue but they are now complemented by studies in other aspects of living cells' function, such as the measurement of cellular stiffness and cell adhesiveness4,5).
This review will discuss some recent contributions of AFM to renal physiology with a focus on monitoring the effect of BP (blood pressure) lowering agents on cellular biological and mechanical responses.
Principle of AFM
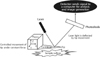
AFM consists of a cantilever with a sharp tip which is used to scan the specimen surface. When the tip is placed near the sample surface, the force between the tip and the sample lead to a deflection of the cantilever (Fig. 1). Laser light is reflected off the back of the deflected cantilever and collected by photodiodes. This interaction between the probe tip and sample surface is then translated into an appropriate image.
In contrast, when the AFM tip is pressed against the cell, the membrane is indented. This mechanical change evokes the distortion of the AFM cantilever, which serves as a spring constant. The cantilever deflection permits force-distance curves of the sample surface (Fig. 2). The slope of the approach curve, the first half of force-distance curve where the tip approaches the sample surface, is used to measure the stiffness of the surface. After the AFM tip has indented the sample surface, the cantilever bends upward (Fig. 2A).
The retraction curve, the second half of force-distance curve where the tip pulls away from the sample surface, is used to determine the adhesion force. Adhesion force between the tip and the surface causes the cantilever to bend downward (Fig. 2B).
A new technical approach to monitor the effects of anti-hypertensive agent by AFM
AFM has become an important biological tool for non-invasive imaging and measuring the mechanical property of cells and materials in renal physiology.
By using AFM, Oberleithner et al.6,7) recently demonstrated that stimulation of the aldosterone receptor changes the endothelial cell stiffness and this phenomenon is related to the endothelial dysfunction observed in high blood pressure. Aldosterone increases plasma membrane roughness, which is detectable only with AFM in cardiomyocytes scanned under physiological conditions8).
Angiotensin II (Ang II) is a potent systemic vasoconstrictor and plays an important role in renal injury by promoting cell apoptosis and inflammation9,10). We recently used AFM to observe Ang II induced dynamic contraction and elastic changes in live and fixed mesangial cells11). We observed structural changes and the stiffening of mesangial cells induced by Ang II. To measure changes in live cell stiffness, force-distance curve measurements were performed on mesangial cells before, and after Ang II application. Ang II activates Ang II type 1 (AT1) receptor at the surface of endothelial, mesangial cell and smooth muscle cells. Activation of the AT1 receptor induces various signaling pathways leading to actin cytoskeletal remodeling and contraction of the cell12). Cuerrier CM et al. recently extended the use of AFM to monitor AT1 receptor activation-induced mechanical responses in renal cells13). The main feature of the AT1 receptor activation is the large upward displacement of the cell membrane with contraction. It is important to mention that these nanoscaled cell contractions and membrane displacements cannot be detected using conventional optical techniques.
Several studies have documented the role of BP lowering agent on cells including endothelial cell and mesangial cell. Recently, AFM was performed to measure the effect of BP lowering agent on cellular stiffness, cell volume and apical surface. Specific β1 receptor blocker, nebivolol, decreases endothelial cell stiffness, which is associated with a significant increase in endothelial cell size14). These morphological and functional modulations observed in endothelial cells may explain the improved endothelial function with nebivolol treatment. A nanoscopy of the cell surface revealed that aldosterone-induced roughness and increase of cell surface are inhibited by spironolactone8). These nano-scaled observations obtained through experiments on individual cells, improve our understanding of the regulation of spironolactone in physiological processes. It could be explained that aldosterone-induced rearrangements of the cytoskeleton may influence the membrane surface of cells. These rearrangements were effectively prevented by spironolactone.
In addition, AFM has been introduced to directly measure the interaction forces between living cells and the AFM tip. We demonstrated for the first time the use of AFM force-distance curves on live mesangial cells to directly monitor changes in surface adhesion and stiffness of cells upon treatment with telmisartan in real time15). The main finding of our study was the observation of an inhibition of the contractile response to Ang II in mesangial cells pre-treated with telmisartan, which was associated with alterations in cell stiffness15). We also demonstrated that telmisartan-pretreated cells showed smaller pull-off forces than did the mesangial cells before treatment15). Measuring the stiffness and adhesive properties of mesangial cells after telmisartan treatment allowed us to explore the roles of Ang II receptor blocker in structural and dynamic changes of mesangial cells. This approach could implicate several advantages in the in vitro experiments using cultured kidney cells.
The ability to image and study the surface of living cells is one of the important tools of AFM (Fig. 3). Fig. 3 shows a time series of topography and three dimensional images of live mesangial cells, showing the movement of mesangial cells at a 60×60 µm scan range. Images are taken before and 20 min after Ang II treatment. Cells moved toward the center. This reflects that Ang II induced contractions might lead to movement of mesangial cell. We could obtain the high quality images of live cells with AFM. However, it is difficult to demonstrate delicate cytoskeletal structures of the cell. With respect to AFM image, we found that the cytoskeletal elements of fixed cells were more prominent than live cells. We performed AFM imaging of fixed cells in a liquid solution to prevent dehydration and to maintain the mesangial cell structure (Fig. 4). The AFM images of fixed mesangial cells show the morphologic characteristics of mesangial cells, including the cytoskeletal structures of actin filaments, other filamentous elements and lamellar structures (Fig. 4). The morphologic characteristics of mesangial cells were well preserved without dehydration. No fixative artifacts were detected. The nucleus height of fixed MCs was 2.51±0.42 µm(n=5) from the bottom of the culture dish. A significant increase in height was observed with Ang II stimulation (n=5, 3.12±0.57 µm). Pretreatment with telmisartan abolished the Ang II effects (n=5, 2.21±0.50 µm).




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download