Abstract
Background
Antibiotic spacers in shoulder periprosthetic joint infection deliver antibiotics locally and provide temporary stability. The purpose of this study was to evaluate differences between stemmed and stemless spacers.
Methods
All spacers placed from 2011 to 2013 were identified. Stemless spacers were made by creating a spherical ball of cement placed in the joint space. Stemmed spacers had some portion in the humeral canal. Operative time, complications, reimplantation, reinfection, and range of motion were analyzed.
Results
There were 37 spacers placed: 22 were stemless and 15 were stemmed. The stemless spacer population was older (70.9 ± 7.8 years vs. 62.8 ± 8.4 years, p = 0.006). The groups had a similar percentage of each gender (stemless group, 45% male vs. stemmed group, 40% male; p = 0.742), body mass index (stemless group, 29.1 ± 6.4 kg/m2 vs. stemmed group, 31.5 ± 8.3 kg/m2; p = 0.354) and Charlson Comorbidity Index (stemless group, 4.2 ± 1.2 vs. stemmed group, 4.2 ± 1.7; p = 0.958). Operative time was similar (stemless group, 127.5 ± 37.1 minutes vs. stemmed group, 130.5 ± 39.4 minutes). Two stemless group patients had self-resolving radial nerve palsies. Within the stemless group, 15 of 22 (68.2%) underwent reimplantation with 14 of 15 having forward elevation of 109° ± 23°. Within the stemmed group, 12 of 15 (80.0%, p = 0.427) underwent reimplantation with 8 of 12 having forward elevation of 94° ± 43° (range, 30° to 150°; p = 0.300). Two stemmed group patients had axillary nerve palsies, one of which self-resolved but the other did not. One patient sustained dislocation of reverse shoulder arthroplasty after reimplantation. One stemless group patient required an open reduction and glenosphere exchange of dislocated reverse shoulder arthroplasty at 6 weeks after reimplantation.
Periprosthetic joint infection (PJI) in shoulder arthroplasty remains a diagnostic and management conundrum. The reported infection rate after primary shoulder arthroplasty is just under 1%1) and it is approximately 5% after primary reverse shoulder arthroplasty.2) Treatment options include antibiotic treatment, irrigation and debridement, single-stage revision, two-stage revision, resection arthroplasty, and arthrodesis.3456789) While there is no definitive standard of care for the treatment of infected shoulder arthroplasty, two-stage revision is often utilized in a similar fashion to the common practice in hip and knee PJI.1011121314) Placement of an antibiotic spacer may be a bridge to a definitive arthroplasty with a two-stage revision or a definitive management option for PJI. Stine et al.15) showed that the use of an articulating spacer was a suitable definitive treatment option in some patients; however, others experienced persistent pain and limited function that led to reimplantation.
The topic of antibiotic spacer types has been well-studied in two-stage revision for hip and knee PJI. Recent analyses have suggested that dynamic spacers have improved functional outcomes and better soft tissue preservation when compared to static spacers.161718) Furthermore, the use of dynamic spacers in two stage-revision knee arthroplasty allows for increased range of motion throughout the treatment with the spacer leading to reduced bone loss and less muscle atrophy without any evidence of significant wear damage.19) In shoulder PJI, there are two main categories of antibiotic spacers utilized in two-stage revisions: stemmed and stemless. The clinical differences between these two implant choices have not yet been studied. For the purpose of this study, any antibiotic spacer that had any cement that entered into the humeral canal was considered a stemmed implant while any implant that was formed by simply creating a sphere of cement that was placed into the joint cavity after debridement was considered stemless. The purpose of this study was to evaluate all patients who underwent antibiotic spacer placement and evaluate differences in outcomes between the stemmed and stemless implants.
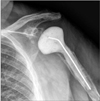
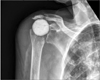
The study was approved by the Institutional Review Board of Thomas Jefferson University Hospital (IRB No. 45 CFR 46.110; #16D.594) and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was waived as this was purely retrospective review without intervention. After approval from the Institutional Review Board, we performed a retrospective analysis of our institutional shoulder arthroplasty database between January 1, 2011 and December 31, 2013. This database identified all primary and revision shoulder arthroplasty cases defined by the International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM) codes. The codes utilized were 79.31 (open reduction of fracture of humerus), 80.01 (arthrotomy for removal of a prosthesis without replacement), 81.80 (total shoulder arthroplasty), 81.81 (shoulder hemiarthroplasty), 81.82 (repair of recurrent dislocation of shoulder), 81.83 (other repair of shoulder, arthroplasty), 81.88 (reverse total shoulder arthroplasty), and 81.97 (revision joint replacement upper extremity). Direct chart review was then performed to identify the subpopulation of patients that underwent placement of an antibiotic spacer. Operative notes and postoperative radiographs were utilized to determine if the antibiotic spacer was stemmed (Fig. 1) or stemless (Fig. 2). Prefabricated implants were not utilized. All spacers were created with tobramycin and vancomycin. All stemless spacers were made by creating a spherical ball of cement that was placed in the joint space. Any antibiotic spacer that had any cement that entered into the humeral canal was considered a stemmed implant. All stemmed spacers were created by fashioning cement into the shape of a stemmed humeral implant, with or without placement of a central, pre-bent wire. Complete medical records were reviewed to determine the total operative time from skin incision to skin closure in each case as well as complications. Operative cultures were reviewed for identification of infecting organism(s). Postoperative clinic notes at 2, 6, and 12 weeks as well as the final clinic note in our electronic medical records were reviewed to determine whether or not patients underwent reimplantation, the timing of reimplantation, and final range of motion after reimplantation. At a minimum of 2 years of clinical follow-up, recurrence of infection and any future reoperations following reimplantation were determined.
Descriptive statistics were utilized to compare the two antibiotic spacer subpopulations. Differences in categorical variables were evaluated by comparison of z-scores of proportions while continuous variables were evaluated by Student paired t-tests. Preoperative variables compared between two spacer subpopulations were age, gender, body mass index (BMI), and age-adjusted Charlson Comorbidity Index (CCI).2021) The operative times of antibiotic spacer placement were then compared between the two groups. The rates of reimplantation (second stage of the two-stage revision) were analyzed. Finally, in patients who underwent reimplantation, range of motion in forward elevation was measured at the latest postoperative visit (minimum 6 months). This range of motion data was directly collected by the surgeon. All statistics were calculated with Microsoft Excel 2013 (Redmond, WA, USA).
Retrospective review of our institutional shoulder arthroplasty database identified 37 patients who underwent antibiotic spacer placement over the study period. There were 22 stemless implants (59.5%) placed and 15 stemmed implants (40.5%). The stemless group had an average age of 70.9 ± 7.8 years (range, 54.6 to 84.7 years), was 45.5% male, had an average BMI of 29.1 ± 6.4 kg/m2 (range, 19.5 to 48.0 kg/m2), and had an average age-adjusted CCI of 4.2 ± 1.2 (range, 2 to 6). There were two intraoperative complications in the stemless group; both were nerve injuries with electromyography-proven radial nerve palsies that self-resolved within 6 months of surgery. The culture data from the stemless group revealed that 12 of 22 (54.5%) had positive cultures: four isolated Propionibacterium acnes (33%), three coagulase-negative Staphylococcus species (CNS, 25%), 2 methicillin-sensitive Staphylococcus aureus (MSSA, 16.7%), one Enterococcus faecalis (8.3%), one Escherichia coli (8.3%), and one with both MSSA and P. acnes (8.3%). In comparison, the stemmed group had an average age of 62.8 ± 8.4 years (range, 48.0 to 81.9 years; p = 0.006), was 40% male (p = 0.742), had an average BMI of 31.5 ± 8.3 kg/m2 (range, 21.5 to 47.6 kg/m2; p = 0.354), and an average age-adjusted CCI of 4.2 ± 1.7 (range, 2 to 8; p = 0.958). Operative time for the stemless group was 127.5 ± 37.1 minutes (range, 62 to 200 minutes) compared to 130.5 ± 39.4 minutes (range, 74 to 188 minutes; p = 0.820) in the stemmed group. There were no intraoperative complications in the stemmed group. The culture data from the stemmed group was available for 14 of 15 patients and revealed that 7 of 14 (50.0%) had positive cultures: four isolated P. acnes (57.1%) and three MSSA (42.8%) (Table 1).
In the entire antibiotic spacer population, 27 of 37 patients (73.0%) underwent revision to definitive arthroplasty. Within the stemless group, 15 of 22 of patients (68.2%) went on to 18 future surgeries (two spacer exchanges, 15 reverse shoulder arthroplasties, and one open reduction of a dislocated reverse shoulder arthroplasty with glenosphere exchange) at an average of 6.8 ± 8.5 months (range, 0.8 to 34.0 months) after the index spacer placement (Table 2). In those stemless patients who were converted to a revision arthroplasty, 14 of 15 (93.3%) had final range of motion data at an average follow-up of 23.2 ± 11.9 months (range, 6.0 to 44.5 months). These patients had an average forward elevation of 109° ± 23° (range, 70° to 150°). The operative time for definitive reimplantation after stemless spacer placement was 143 ± 42 minutes (range, 89 to 236 minutes). There were no intraoperative complications in reimplantation within the stemless group. Intraoperative cultures were drawn for 14 of 15 reimplantations in the stemless group with 3 of 15 (20.0%) having at least one positive culture: one had negative cultures, but P. acnes were found on gram stain (this patient had negative cultures at the index spacer placement), one grew P. acnes (this patient had P. acnes at the index spacer placement), and one grew CNS (this patient had negative cultures at the index spacer placement). The first of these patients was treated as contaminant, while the other two were treated with oral antibiotics for 6 weeks. None of these three patients had clinical evidence of infection postoperatively. One stemless patient underwent reoperation for a dislocated reverse shoulder arthroplasty at 6 weeks after reimplantation. This patient required an open reduction with glenosphere exchange. With the exception of one reoperation, no patient required future reoperation at an average follow-up of 3.6 ± 1.0 years (range, 1.5 to 5.2 years).
Within the stemmed group, 14 of 15 (93.3%) went on to 15 future surgeries (two spacer revisions, one resection arthroplasty, one long-stemmed hemiarthroplasty, four total shoulder arthroplasties, and seven reverse shoulder arthroplasties) with 12 of 15 (80.0%, p = 0.427) undergoing definitive reimplantation at an average of 2.4 ± 0.7 months (range, 0.7 to 4.4 months) after the index spacer placement (Table 3). In those stemmed patients who were converted to a revision arthroplasty, 8 of 12 (66.7%) had final range of motion data at an average follow-up of 18.8 ± 8.5 months (range, 6.4 to 35.8 months). These patients had an average forward elevation of 94° ± 43° (range, 30° to 150°; p = 0.300). The operative time for definitive reimplantation after stemmed spacer placement was 154 ± 70.1 minutes (range, 57 to 304 minutes; p = 0.653). There were three complications during reimplantation in the stemmed spacer group. One patient was found to have a dislocated reverse shoulder arthroplasty in the recovery room on postoperative radiographs at which point the patient was brought back to the operating room for closed reduction. Two patients were found to have atrophy and loss of contractility of the anterior and middle deltoid. One had a confirmed diagnosis of axillary nerve palsy on electromyography that self-resolved within 6 months, while the other refused electromyography and had an incomplete return of function. Intraoperative cultures were recorded for 11 of 12 reimplantations in the stemmed group with 3 of 11 (27.3%) having at least one positive culture. In these three patients, one grew E. coli (one colony in broth only; grew P. acnes at the index spacer placement) and two grew an unspecified bacillus species (both one colony in broth only; both were negative at the index spacer placement). These were all treated as contaminant without clinical evidence of infection postoperatively. Following reimplantation from a stemmed spacer, there were no further reoperations performed at an average follow-up of 4.0 ± 0.8 years (range, 2.4 to 5.2 years). There were no clinical reinfections in either the stemless or stemmed population.
The role of two-stage revision in management of shoulder PJI has been well-described.3456789) Spacer placement is a viable option either as a bridge to reimplantation or as a definitive treatment modality. In our study, over three quarters of patients went on to have revision arthroplasty while the rest retained the spacer as a definitive implant. In both groups, the infection was adequately treated as there were no clinical signs of recurrent infections. Additionally, there was only one reoperation after reimplantation (glenosphere exchange for a reverse shoulder arthroplasty at 6 weeks after reimplantation), and there was no statistical difference between the stemmed and stemless spacers in terms of eventual reimplantation.
In hip and knee PJI, recent analyses found improved functional outcomes and better soft tissue preservation of dynamic spacers when compared to static spacers.161718) Prior to this study, there has not been an analysis on the ideal antibiotic spacer design in shoulder PJI. One advantage of stemless spacers that we expected was reduced operative time since the implant can be made from the beginning of the case and is easily inserted in the joint. However, we found similar operative times for implantation of both designs. It is likely that the operative time of complex shoulder PJI cases is driven by a number of technical variables, such as ease of stem excision, and that the effect of spacer type is negligible. Additionally, operative time of reimplantation was similar between the groups. This suggests that both implant types equally maintained the joint space and both are equally easy to remove upon revision surgery. Finally, the similar range of motion data after definitive reimplantation suggests that the choice of a stemmed or stemless implant does not have a major impact on the final functional outcome. While neither group returned to full range of motion, they did achieve similar forward elevation to that previously described for revision of hemiarthroplasty to reverse total shoulder arthroplasty.2223)
There were five total complications in the entire study group, two with index stemless spacer placement and three with reimplantation after stemmed implant placement. These complications included four neurologic injuries and one reverse shoulder arthroplasty dislocation. There were 70 total surgeries performed in our entire population which gives a neurologic injury rate of 5.7%, three of which completely resolved and one of which incompletely resolved. The literature reports a rate of neurologic injury of 1% to 4.3% in anatomic total shoulder arthroplasty2425) and from 1.7% to 11.6% in reverse shoulder arthroplasty262728) with revision surgery being a risk factor for neurologic injury.29) The rate of neurologic injury in our patient population is within the range we expected given the complex nature of these cases. Three of these neurologic deficits fully resolved while one partially resolved, consistent with previous reports of these injuries being stretch neuropraxias rather than transection injuries.2527) Regarding bacterial cultures at the time of reimplantation, all patients that had one or less positive culture at the time of reimplantation were treated as a contaminant while those with two or more positive cultures were treated as true positive cultures per the protocol described by Frangiamore et al.30) The two patients that met this criteria of culture positivity at the time of reimplantation were given 6 weeks of postoperative antibiotics. There were no reinfections after reimplantation in this study.
The findings of this study must be considered in the context of the limitations. First, the majority of our patients went on to have revision arthroplasty, which did not allow us to further study the differences between stemmed and stemless spacers as a final treatment option. Also, we did not have sufficient patient reported outcomes to fully analyze functional differences. While similar outcomes in forward elevation may provide a general idea of functional level, the availability of other outcomes, such as patientspecific functional results, would have been more substantial. Additionally, this is a purely retrospective study and therefore we can only determine associative relationships rather than speculate on causality. These were also very complex patients from both medical and technical perspectives. Therefore, retrospective analysis of the details of their clinical course is difficult. In order to mitigate this weakness, all of the patient charts were reviewed directly in their entirety instead of simply relying on the institutional database. Finally, because this was a retrospective study, the patient numbers were set without power analysis. Therefore, the nonsignificant trend of a higher reimplantation rate in patients with stemmed spacers may simply be a result of an underpowered study rather than truly nonsignificant.
This study offers the first comparison between patients with stemmed antibiotic spacers and stemless antibiotic spacers in shoulder arthroplasty. The patient populations that underwent stemmed and stemless antibiotic spacer placement were statistically similar in complication rate, final reimplantation rate, operative time, and final active forward elevation. When analyzing all antibiotic spacers, over 70% were converted to revision arthroplasties, after which there was one reoperation and no clinical reinfections. The results of this study do not suggest superiority of either stemmed or stemless antibiotic spacers. Future prospective analysis may determine differences in outcomes not observed in this study.
Figures and Tables
Table 1
Comparison of Stemmed and Stemless Groups

Table 2
Surgical Variables for All Patients That Underwent Stemless Spacer Placement

Table 3
Surgical Variables for All Patients That Underwent Stemmed Spacer Placement

References
1. Padegimas EM, Maltenfort M, Ramsey ML, Williams GR, Parvizi J, Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elbow Surg. 2015; 24(5):741–746.

2. Florschutz AV, Lane PD, Crosby LA. Infection after primary anatomic versus primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015; 24(8):1296–1301.

3. Clare DJ, Wirth MA, Groh GI, Rockwood CA Jr. Shoulder arthrodesis. J Bone Joint Surg Am. 2001; 83-A(4):593–600.

4. Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg Br. 2004; 86(1):65–69.

5. Ince A, Seemann K, Frommelt L, Katzer A, Loehr JF. Onestage exchange shoulder arthroplasty for peri-prosthetic infection. J Bone Joint Surg Br. 2005; 87(6):814–818.

6. Jerosch J, Schneppenheim M. Management of infected shoulder replacement. Arch Orthop Trauma Surg. 2003; 123(5):209–214.

7. Levy JC, Triplet J, Everding N. Use of a functional antibiotic spacer in treating infected shoulder arthroplasty. Orthopedics. 2015; 38(6):e512–e519.

8. Rispoli DM, Sperling JW, Athwal GS, Schleck CD, Cofield RH. Pain relief and functional results after resection arthroplasty of the shoulder. J Bone Joint Surg Br. 2007; 89(9):1184–1187.

9. Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001; (382):206–216.

10. Fitzgerald SJ, Hanssen AD. Surgical techniques for staged revision of the chronically infected total knee arthroplasty. Surg Technol Int. 2011; 21:204–211.
11. Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004; (427):79–85.

12. Aggarwal VK, Rasouli MR, Parvizi J. Periprosthetic joint infection: current concept. Indian J Orthop. 2013; 47(1):10–17.

13. Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010; 33(9):659.

14. Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003; 85-A:Suppl 1. S75–S80.

15. Stine IA, Lee B, Zalavras CG, Hatch G 3rd, Itamura JM. Management of chronic shoulder infections utilizing a fixed articulating antibiotic-loaded spacer. J Shoulder Elbow Surg. 2010; 19(5):739–748.

16. Chiang ER, Su YP, Chen TH, Chiu FY, Chen WM. Comparison of articulating and static spacers regarding infection with resistant organisms in total knee arthroplasty. Acta Orthop. 2011; 82(4):460–464.

17. Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis: the Ranawat Award. Clin Orthop Relat Res. 2000; (380):9–16.
18. Pietsch M, Wenisch C, Traussnig S, Trnoska R, Hofmann S. Temporary articulating spacer with antibiotic-impregnated cement for an infected knee endoprosthesis. Orthopade. 2003; 32(6):490–497.
19. Jaekel DJ, Day JS, Klein GR, Levine H, Parvizi J, Kurtz SM. Do dynamic cement-on-cement knee spacers provide better function and activity during two-stage exchange? Clin Orthop Relat Res. 2012; 470(9):2599–2604.

20. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004; 57(12):1288–1294.

21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–383.

22. Dezfuli B, King JJ, Farmer KW, Struk AM, Wright TW. Outcomes of reverse total shoulder arthroplasty as primary versus revision procedure for proximal humerus fractures. J Shoulder Elbow Surg. 2016; 25(7):1133–1137.

23. Werner BS, Boehm D, Gohlke F. Revision to reverse shoulder arthroplasty with retention of the humeral component. Acta Orthop. 2013; 84(5):473–478.

24. Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015; 8(1):83–91.

25. Ladermann A, Lubbeke A, Melis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011; 93(14):1288–1293.

26. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007; 89(4):516–520.
27. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011; 19(7):439–449.

28. Ladermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009; 18(4):588–595.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download