This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The role of ultrasound in the thoracic spine has been underappreciated, partly because of the relative efficacy of the landmark-guided technique and the limitation of imaging through the narrow acoustic windows produced by the bony framework of thoracic spine. The aim of this study was to make a comparison between the 12th rib and the spinous process of C7 as a landmark for effective ultrasound-guided target segment identification in the thoracic spine.
Methods
Ultrasonography of 44 thoracic spines was performed and the same procedure was carried out 1 week later again. The target segments (T3–4, T7–8, and T10–11) were identified using the 12th rib (group 1) or the spinous process of C7 (group 2) as a starting landmark. Ultrasound scanning was done proximally (group 1) or distally (group 2) toward the target transverse process and further medially and slightly superior to the target thoracic facet. Then, a metal marker was placed on the T3–4, T7–8, and T10–11 and the location of each marker was confirmed by fluoroscopy.
Results
In the total 132 segments, sonographic identification was confirmed to be successful with fluoroscopy in 84.1% in group 1 and 56.8% in group 2. Group 1 had a greater success rate in ultrasound-guided target segment identification than group 2 (p = 0.001), especially in T10–11 (group 1, 93.2%; group 2, 43.2%; p = 0.001) and T7–8 (group 1, 86.4%; group 2, 56.8%; p = 0.002). The intrarater reliability of ultrasound-guided target segment identification was good (group 1, r = 0.76; group 2, r = 0.82), showing no difference between right and left sides. Ultrasound-guided target segment identification was more effective in the non-obese subjects (p = 0.001), especially in group 1.
Conclusions
Ultrasound-guided detection using the 12th rib as a starting landmark for scanning could be a promising technique for successful target segment identification in the thoracic spine.
Go to :

Keywords: Thoracic spine, Ultrasonography, Nerve block
Although less common than lumbar and cervical spinal pain, the prevalence of thoracic spinal pain in the general population is 13% to 15% in the literature, and it can be as disabling as lumbar or cervical spinal pain.
12) Thoracic spinal nerve block has been commonly used to mitigate pain of the thoracic vertebrae and thoracic cage and pain after breast surgery.
34) Recently, excellent results of pain control for thoracic pain accompanying osteoporotic thoracic vertebral compression fracture have been reported.
56) In addition, with an increasing frequency and indications for thoracic spinal nerve block, attention and research have been more focused on ultrasound-guided thoracic spinal nerve block.
Ultrasound of the thoracic spine has several advantages over traditional radiographic imaging, such as absence of radiation, direct visualization of neurovascular and soft tissue structures, real-time visualization of needle passage toward the intended target, and the ability to perform dynamic imaging. However, it has limitations, including the difficulty of imaging through narrow acoustic windows produced by the skeletal framework and identifying the target segment due to the relatively small image compared to fluoroscopy. Mostly, ultrasound-guided thoracic spinal nerve block is performed with the landmarkguided technique; however, it does not assure that the ultrasonographically identified segment is identical to the actual pathological segment. For this reason, thoracic spinal nerve block is performed at multiple segments including not only the clinically painful segment but also the proximal and distal adjacent segments.
7) Therefore, previous studies on ultrasound-guided thoracic spinal block suggested only the accuracy of contrast material injection at the target facet joint or the paravertebral space as a factor associated with the cause of pain, not the accuracy of target segment identification.
8910) However, with the expanding indications for thoracic spinal nerve block, there has been a growing expectation on favorable results of ultrasound landmark-guided block performed at the definite target segment for conditions, such as facet syndrome associated with thoracic vertebral compression fracture.
There are two kinds of anatomical landmarks used as a starting point for ultrasound-guided target segment identification in the thoracic vertebra: the 12th rib
8) and the spinous process of C7.
1011) The aim of this study was to compare the efficacy of the two landmarks for ultrasoundguided target segment identification in the thoracic spine.
METHODS
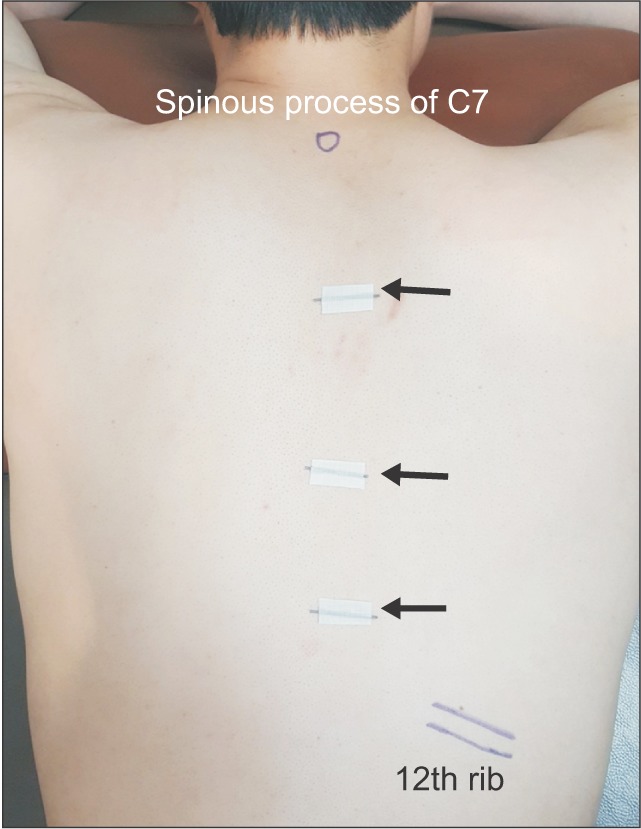
This study was conducted on 22 volunteers without history of spine surgery. The mean age of participants was 30.5 years (range, 23 to 40 years), and there were all male volunteers. The participants fully understood the ultrasound image would be rechecked with C-arm fluoroscopy after marking the target thoracic segment using ultrasound. Ultrasound examination was performed on a total of 66 segments of 22 participants, including T3–4, T7–8, and T10–11 with two different starting landmarks, spinous process of C7 and 12th rib. The same procedure was repeated on the contralateral side of the 66 segments in a week. Thus, ultrasound examination was performed on a total of 132 segments.
After laying the participant on a radiolucent table in prone position, we positioned a high-resolution linear probe (12-MHz SONOs, Logiq S7 Expert; GE Healthcare, Wauwatosa, WI, USA) at the anatomical landmark. The target segments were T3–4, T7–8, and T10–11 in all participants. We divided the two groups according to which anatomical landmark was used for identification of the segments: the 12th rib in group 1 and the spinous process of C7 in group 2.
In group 1, we found the 12th rib using the linear probe in the sagittal section. Then, the probe was moved medially to check the transverse process of T12. Subsequently, ultrasound scanning was done proximally toward the target transverse process and further medially and slightly superior to the target thoracic facet. Finally, when the target facet joint was identified (
Fig. 1), a metal marker was placed at the center of the probe (
Fig. 2). Subsequently, at T10–11, T7–8, and T3–4, an anteroposterior radiograph of the thoracic spine was obtained using fluoroscopy without changing the position of the participant to maintain the same position as in the ultrasound-guided target segment identification. The location of the metal markers was confirmed on the thoracic radiograph. Successful target segment identification was defined by the following two criteria: the metal marker was placed on the target segment and on the target facet joint (
Fig. 3).
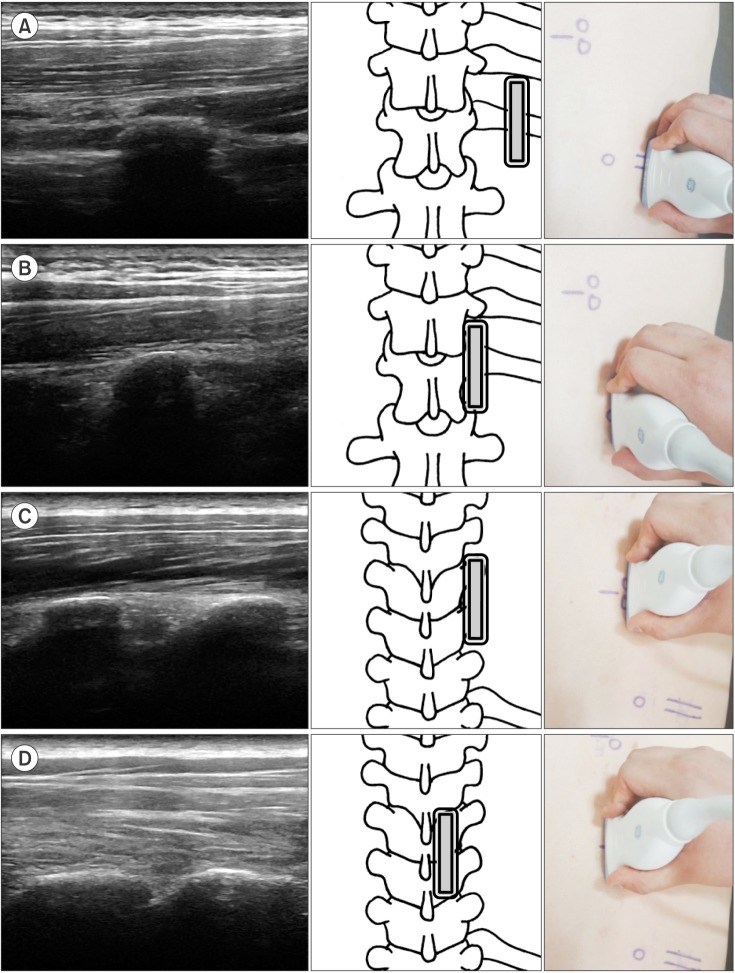 | Fig. 1Ultrasound images of the detection method using the 12th rib as a starting landmark. The double-lined rectangle represents the ultrasound probe. (A) The 12th rib in the sagittal view. (B) The transverse process of T12 was found medial to the 12th rib. (C) The probe was moved proximally to the transverse process of T7 and T8. (D) The facet joint of T7–8 was found medially and slightly proximal to the transverse process.
|
 | Fig. 2Metal makers (black arrows) placed at the target location identified with sonography.
|
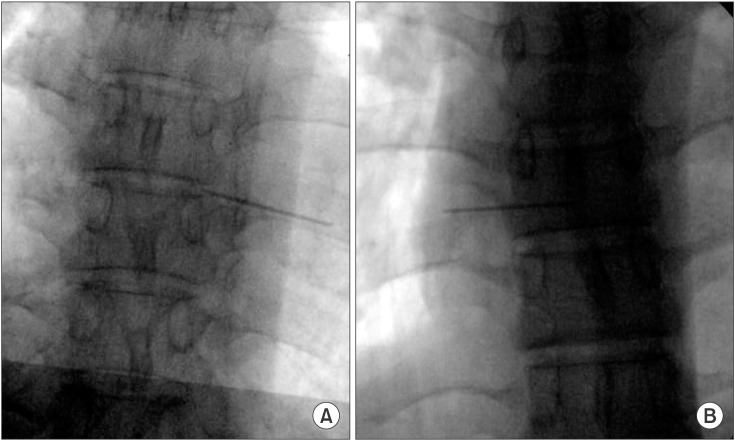 | Fig. 3Validation by C-arm fluoroscopy. (A) Successful case: the metal marker was accurately located at the facet joint of the target segment. (B) Failure case: the metal marker was not accurately located at the facet joint of the target segment.
|
In group 2, we found the spinous process of C7 with the linear probe in the sagittal section. The probe was moved laterally to check the transverse process of T1. Then, ultrasound scanning was done distally toward the target transverse process and further medially and slightly superior to the target thoracic facet joint (
Fig. 4). A metal marker was placed on the T3–4, T7–8, and T10–11 and the location of markers was confirmed by fluoroscopy.
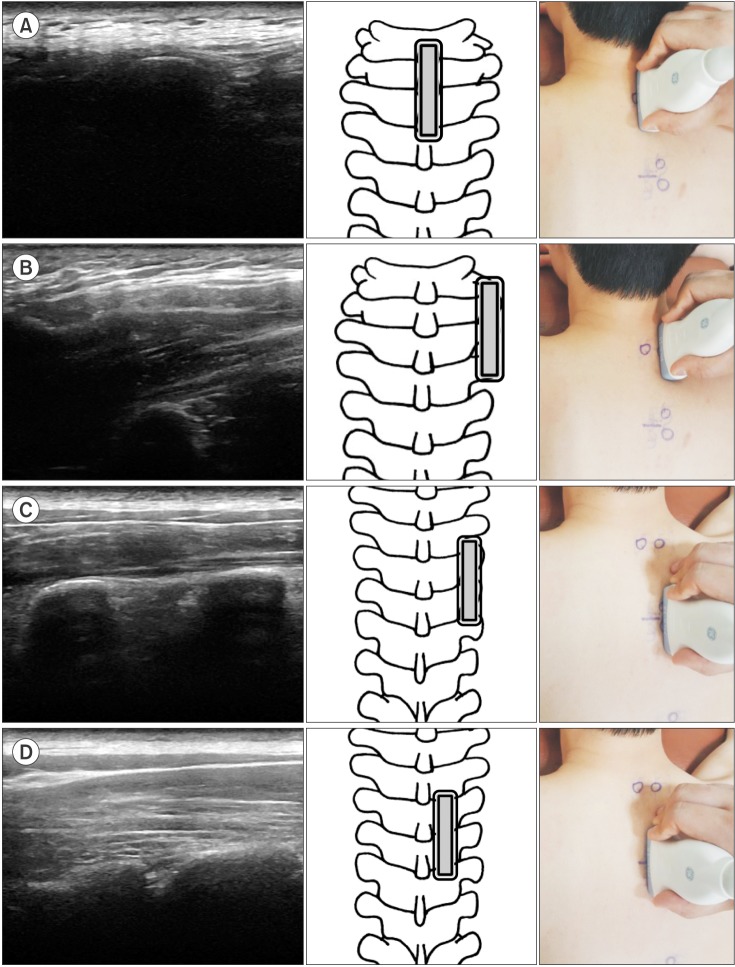 | Fig. 4Ultrasound images of the detection method using the spinous process of C7 as a starting landmark. The double-lined rectangle represents the ultrasound probe. (A) The spinous process of C7 in the sagittal view. (B) The transverse process of T1 was found lateral to the spinous process of C7. (C) The probe was moved distally to the transverse process of T3 and T4. (D) The facet joint of T3–4 was found medially and slight proximal to the transverse process.
|
In a week later, the same procedure was performed on the contralateral side of all participants. All procedures were done by the same physician (YSC). The total target segments (T3–4, T7–8, and T10–11) and the target facet joints of the total 132 segments were identified.
Intrarater reliability was assessed by comparing the values obtained from the left and right sides. Additionally, based on the assumption that the accuracy would be decreased among obese people due to thick fat tissue, we subdivided the participants in each group according to body mass index (BMI) into a non-obese group (BMI < 25.0 kg/m2) and an obese group (BMI ≥ 25.0 kg/m2) and compared the accuracy of target segment identification. The non-obese group and obese group did not have difference in age and height.
Demographical features were compared using the Mann-Whitney U-test. The accuracy of target segment identification according to the landmark and obesity was assessed using the chi-square test. Analysis of the left and right side examination results was compared using intraclass correlation coefficients. Statistical analysis was carried out using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) and p < 0.05 was considered statistically significant.
Go to :

RESULTS
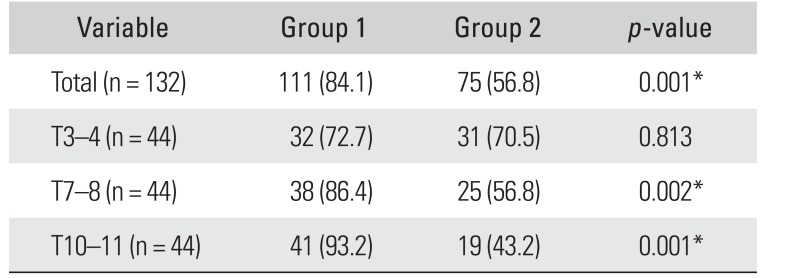
Of the total 132 segments, overall target segment identification in the thoracic spine was confirmed to be successful by fluoroscopy in 84.1% in group 1 and 56.8% in group 2. Overall, ultrasound-guided target segment identification was more effective in group 1 than group 2 (
p = 0.001), especially in T10–11 (group 1, 93.2%; group 2, 43.2%;
p = 0.001) and T7–8 (group 1, 86.4%; group 2, 56.8%;
p = 0.002). However, there was no significant difference between groups in T3–4 (group 1, 72.7%; group 2, 70.5%;
p = 0.813) (
Table 1).
Table 1
Validation of Ultrasound-Guided Thoracic Target Segment Identification Confirmed by Fluoroscopy
|
Variable |
Group 1 |
Group 2 |
p-value |
|
Total (n = 132) |
111 (84.1) |
75 (56.8) |
0.001*
|
|
T3–4 (n = 44) |
32 (72.7) |
31 (70.5) |
0.813 |
|
T7–8 (n = 44) |
38 (86.4) |
25 (56.8) |
0.002*
|
|
T10–11 (n = 44) |
41 (93.2) |
19 (43.2) |
0.001*
|

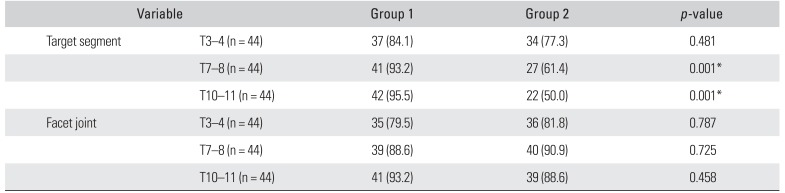
The efficacy of the two landmarks for sonographic target segment identification was also evaluated according to each criterion for success. Based on the success criteria for target segment identification, group 1 had a significantly higher success rate than group 2 in T7–8 and T10–11 (
p = 0.001). On the other hand, there was no difference between the groups for target facet joint identification (T3–4,
p = 0.787; T7–8,
p = 0.725; and T10–11,
p = 0.458) (
Table 2). The starting landmarks had greater influence on successful identification of the target segment than the facet joint.
Table 2
Accuracy of Ultrasound-Guided Identification of the Target Segment and Facet Joint
|
Variable |
Group 1 |
Group 2 |
p-value |
|
Target segment |
T3–4 (n = 44) |
37 (84.1) |
34 (77.3) |
0.481 |
|
T7–8 (n = 44) |
41 (93.2) |
27 (61.4) |
0.001*
|
|
T10–11 (n = 44) |
42 (95.5) |
22 (50.0) |
0.001*
|
|
Facet joint |
T3–4 (n = 44) |
35 (79.5) |
36 (81.8) |
0.787 |
|
T7–8 (n = 44) |
39 (88.6) |
40 (90.9) |
0.725 |
|
T10–11 (n = 44) |
41 (93.2) |
39 (88.6) |
0.458 |

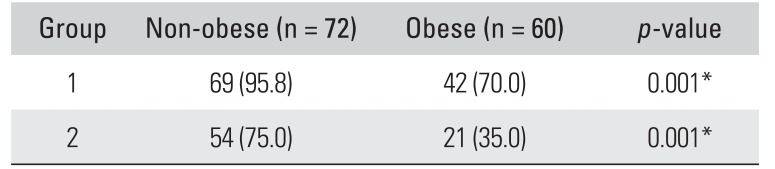
There was good intrarater reliability of ultrasoundguided target segment identification (group 1,
r = 0.76; group 2,
r = 0.82), showing no significant difference between the right and left side data. According to the influence of BMI, the incidence of obtaining successful results in the obese group was 70.0% in group 1 and 35.0% in group 2 of the total 60 segments. On the other hand, the incidence in the non-obese group was 95.8% in group 1 and 75.0% in group 2 of the total 72 segments. Thus, ultrasound-guided target segment identification was more effective in the non-obese participants especially group 1 (
p = 0.001) (
Table 3).
Table 3
Accuracy of Ultrasound-Guided Thoracic Target Segment Identification According to Obesity
|
Group |
Non-obese (n = 72) |
Obese (n = 60) |
p-value |
|
1 |
69 (95.8) |
42 (70.0) |
0.001*
|
|
2 |
54 (75.0) |
21 (35.0) |
0.001*
|

Go to :

DISCUSSION
Extensive research has been conducted to advanceknowledge and understanding of ultrasonographic target segment identification of cervical and lumbar vertebrae.
121314) On the other hand, there is a paucity of research in the literature on the target segment identification of the thoracic spine. Most studies have focused only on the efficacy of the procedure and anatomical structure of the target region, such as the facet joint and paravertebral space.
The morphology of 12 thoracic vertebrae varies depending on the location of the thoracic vertebrae. The first four thoracic vertebrae (T1–4) are similar to the cervical vertebrae, and the four lowest thoracic vertebrae (T9–12) are similar to the lumbar spine in some respects. In addition, the thoracic spine has a longer working length and lack of surface landmarks, making it difficult to identify target segments using ultrasound.
Methods for target segment identification in the thoracic spine have not been documented sufficiently. Stulc et al.
8) have described a target segment identification method using the 12th ribs. First, a transducer was moved upward from the upper lumbar area to locate the 12th rib. Then, the transducer was moved medially through the transverse process of T12 and L1 to find the facet joint and then upward to the target segment. Meanwhile, Deimel et al.
10) and Marhofer et al.
11) described a segment identification method using the spinous process of C7. First, the spinous process of C7 was checked with a transducer, which was moved downward to the spinous process of the target segment. Then, the transducer was moved laterally toward the target region.
Stulc et al.
8) reported that 80% of contrast material was accurately injected into the facet joint of 20 segments of one cadaveric specimen when the 12th rib was used as a starting landmark. O Riain et al.
9) also reported an accuracy of 90% in 10 segments of one cadaver. On the other hand, Deimel et al.
10) reported that 68.8% of contrast material was accurately injected at the costotransverse joint of 16 segments of one cadaver when the spinous process of C7 was used as a landmark. However, since these studies are based on the evaluation of only one cadaver, it appears difficult to generalize these results.
In this
in vivo study, the accuracy of target segment identification was 84.1% when the 12th rib was used as a starting landmark and 56.8% when the spinous process of C7 was used. We had lower successful results with the spinous process of C7 than in the study of Deimel et al.
10) The starting landmarks had greater influence on successful identification of the target segment than the facet joint. These results suggest that the low accuracy in group 2 was associated with level miscounting due to misidentification of the spinous process of C7 as C6 or T1. According to Galiano et al.,
15) identification of C7 is technically demanding and limited in accuracy due to the similar shape of the adjacent segments. The thoracic transverse processes arise posterior to the articular processes and articulate with the corresponding rib. The T12 rib is an identifying feature of the transition between L1 and T12 vertebrae as an anatomical starting landmark for sonography and can be used in conjunction with the “counting-up” approach from the T12–L1 junction to determine the target segment. The 12th ribs are known to be congenitally absent in some population.
16) However, considering that patients who have thoracic pain are subjected to radiographic examination, if the 12th ribs are confirmed absent by radiography, the 11th rib can be used as a landmark for ultrasonographic identification. In other words, we can know whether a patient does not have the 12th rib before ultrasonographic examination.
In obese patients, structures are often less distinguishable because of attenuation that occurs as the ultrasound waves travel a greater distance to pass through thicker soft tissues. Also, the phase aberration effect caused by various velocities of ultrasonic waves generated by the irregular shape of the fat layer has been reported.
17) It is known to be difficult to perform ultrasound-guided intervention for obese patients due to the noise in the ultrasound image and deteriorated resolution of deep structures.
18) Rauch et al.
18) reported only 62% of accuracy of lumbar spinal medial branch block among obese patients. In this study, we also noted ultrasound-guided target segment identification was more effective in non-obese persons. Ueshima et al.
19) suggested the use of around probe that is known to facilitate better visibility in obese patients. In addition, the advancement of imaging technology such as compound imaging and tissue harmonic imaging can complement the deterioration of imaging quality, and recent studies suggest the feasibility of ultrasonography in obese populations.
2021)
This study has some limitations. First, it has a small number of all male participants. Second, this study was not carried out on patients who had actual thoracic disease and it only evaluated the accuracy of identifying the target thoracic segment. Third, there was no evaluation on the effect or complications that can occur in clinical settings. Therefore, further studies should be performed to determine the efficacy of target segment identification in thoracic block in patients with actual thoracic disease.
Upward counting with the 12th rib as a starting landmark offered more successful results in ultrasound-guided target segment identification than downward counting from the spinous process of C7. The starting landmarks had greater influence on successful identification of the target segment than the facet joint. In addition, ultrasound-guided target segment identification was more effective in non-obese people, especially when the 12th rib was used as a starting landmark. These results suggest canning proximally from the 12th rib toward the target transverse process can be a promising technique for ultrasound-guided target segment identification in the thoracic spine.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download