This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Musculoskeletal involvement in melioidosis is often seen in conjunction with a disseminated illness. Recent reports suggest that operative management of musculoskeletal melioidosis has favourable results. The purpose of this study was to review the patient profile and clinical outcomes of Burkholderia pseudomallei infection in the musculoskeletal system.
Methods
Hospital records of 163 patients who were diagnosed to have B. pseudomallei infection between January 2009 and December 2014 were reviewed. Patients underwent surgical and nonsurgical management depending upon the tissue of involvement. Epidata software was used to record the data. The SPSS ver. 17.0 was used for analysis.
Results
Eighteen out of 24 patients who had musculoskeletal melioidosis were available for follow-up. Septic arthritis, osteomyelitis, and intramuscular abscess were the common diagnosis, with 6 patients in each group. Twelve patients required surgical intervention. All patients received a full course of parenteral ceftazidime followed by oral doxycycline and co-trimoxazole. Two out of 6 patients (33.3%) died among those who had nonsurgical management as compared to none in the group who had surgical management. This was significant at 10% level of significance (p = 0.098). The rest were followed up for a minimum of 1 year with no evidence of disease recurrence.
Conclusions
This series describing musculoskeletal involvement in melioidosis is the largest such study from a recently recognized ‘endemic’ region. Of importance are the patterns of musculoskeletal involvement, pitfalls in diagnosis and adequate clinical response with timely diagnosis and appropriate surgical management.
Go to :

Keywords: Burkholderia pseudomallei, Synovitis, Debridement, Osteomyelitis
Melioidosis is an infection caused by
Burkholderia pseudomallei, a fastidious, facultative, intracellular gram-negative microorganism. It spreads by a myriad of routes (direct contact with contaminated water or soil, inhalation, ingestion, and cutaneous inoculation),
1) resulting in a multitude of clinical manifestations. First described in 1912 by Whitmore and Krishnaswami,
2) melioidosis is considered endemic in Thailand, Australia, and parts of Southeast Asia, which account for majority of published data.
34) In India the earliest report was in the year 1990.
5) Subsequent reports have stressed that although India is not a region of high endemicity, melioidosis is prevalent and under reported probably due to low index of suspicion and lack of sufficient diagnostic facilities.
6) The musculoskeletal system is often affected in conjunction with a disseminated illness with rates of involvement being 4% to 12%.
789) In this study we describe the demographic profile and clinical outcomes of patients treated in Christian Medical College and Hospital for
B. pseudomallei infection of the musculoskeletal system. We hypothesized that early detection and timely surgical intervention would improve results in musculoskeletal melioidotic infection.
METHODS
The Institutional Review Board of Christian Medical College and Hospital approved this study (No. 9184). Patients with musculoskeletal involvement of B. pseudomallei infection, either primarily or as a part of a disseminated disease, were considered in the study population. Exclusion criteria comprised patients with polymicrobial infections and patients with pyrexia of unknown origin in whom no organism was isolated in either blood or tissue cultures. We retrospectively reviewed outpatient charts, discharge summaries and medical report of patients who were diagnosed to have B. pseudomallei infection from January 2009 to December 2014. Patient's demographic data, comorbidities, pattern of clinical manifestations, details of medical and orthopaedic treatment were documented. Microbiological and molecular confirmation of the organism was performed in all patients. Histopathology was done on patients in whom tissue material was obtained at the time of diagnostic or surgical intervention.
Epidata software was used to record the data. The SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for analysis. Means with standard deviations were presented for continuous variables. Categorical variables were presented using frequencies and percentages. Prevalence of musculoskeletal involvement among meloidosis infection was expressed as percentage with 95% confidence interval (CI). Fisher exact test was applied to compare the proportion of final outcome (dead/cured) among those who had surgical or nonsurgical treatment.
Go to :

RESULTS
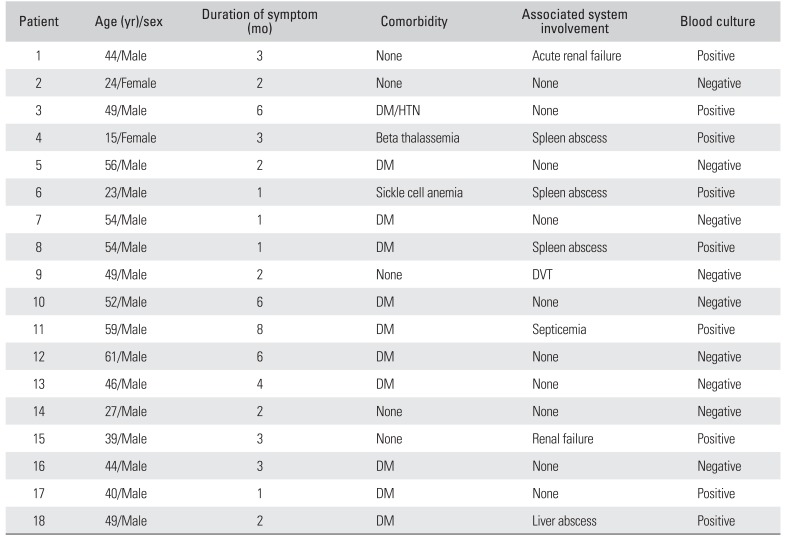
Of 163 patients with culture proven meloidosis infection, 14.7% (24/163; 95% CI, 9.7% to 21.1%) had musculoskeletal involvement. Eighteen patients (16 males and 2 females) were available for follow-up. The average age of the patients in this series was 46 years (range, 15 to 61 years). The average duration of symptoms before diagnosis was 3 months (range, 1 to 9 months). Seven patients (38%) were started on empirical antitubercular therapy by their primary treating physician prior to referral to our centre. Ten patients (55%) had only musculoskeletal involvement, while in 8 patients (44%) who presented with sepsis, musculoskeletal involvement was diagnosed on secondary survey. Eleven (61%) were uncontrolled diabetics while 7 (38%) had no obvious comorbidity. Interestingly, 13 patients were sedentary office workers from urban areas. Nine patients (50%) had at least one positive blood culture. Patient demographics and clinical profile are shown in
Table 1.
Table 1
Demographic and Clinical Profile of Patients with Musculoskeletal Melioidosis
|
Patient |
Age (yr)/sex |
Duration of symptom (mo) |
Comorbidity |
Associated system involvement |
Blood culture |
|
1 |
44/Male |
3 |
None |
Acute renal failure |
Positive |
|
2 |
24/Female |
2 |
None |
None |
Negative |
|
3 |
49/Male |
6 |
DM/HTN |
None |
Positive |
|
4 |
15/Female |
3 |
Beta thalassemia |
Spleen abscess |
Positive |
|
5 |
56/Male |
2 |
DM |
None |
Negative |
|
6 |
23/Male |
1 |
Sickle cell anemia |
Spleen abscess |
Positive |
|
7 |
54/Male |
1 |
DM |
None |
Negative |
|
8 |
54/Male |
1 |
DM |
Spleen abscess |
Positive |
|
9 |
49/Male |
2 |
None |
DVT |
Negative |
|
10 |
52/Male |
6 |
DM |
None |
Negative |
|
11 |
59/Male |
8 |
DM |
Septicemia |
Positive |
|
12 |
61/Male |
6 |
DM |
None |
Negative |
|
13 |
46/Male |
4 |
DM |
None |
Negative |
|
14 |
27/Male |
2 |
None |
None |
Negative |
|
15 |
39/Male |
3 |
None |
Renal failure |
Positive |
|
16 |
44/Male |
3 |
DM |
None |
Negative |
|
17 |
40/Male |
1 |
DM |
None |
Positive |
|
18 |
49/Male |
2 |
DM |
Liver abscess |
Positive |

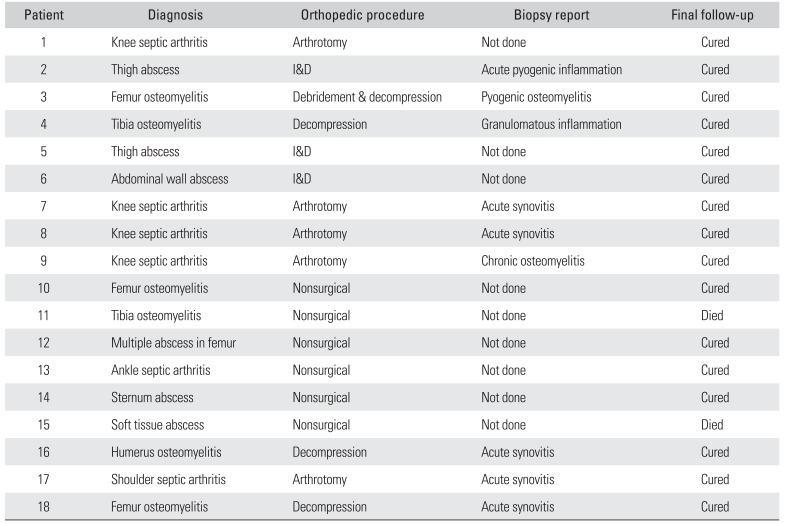
Microbiological confirmation was the basis of diagnosis. Histopathological examination was performed in 9 patients (50%). Findings ranged from acute/chronicosteomyelitis/synovitis to necrotizing granulomatous inflammation. Osteomyelitis, septic arthritis, and intramuscular abscess were the common presentations with each group having 6 patients diagnosed with melioidotic infection. Common regions of involvement were the distal femur and knee joint (
Table 2).
Table 2
Clincal Diagnosis, Procedure Performed, and Follow-up Data of Patients
|
Patient |
Diagnosis |
Orthopedic procedure |
Biopsy report |
Final follow-up |
|
1 |
Knee septic arthritis |
Arthrotomy |
Not done |
Cured |
|
2 |
Thigh abscess |
I&D |
Acute pyogenic inflammation |
Cured |
|
3 |
Femur osteomyelitis |
Debridement & decompression |
Pyogenic osteomyelitis |
Cured |
|
4 |
Tibia osteomyelitis |
Decompression |
Granulomatous inflammation |
Cured |
|
5 |
Thigh abscess |
I&D |
Not done |
Cured |
|
6 |
Abdominal wall abscess |
I&D |
Not done |
Cured |
|
7 |
Knee septic arthritis |
Arthrotomy |
Acute synovitis |
Cured |
|
8 |
Knee septic arthritis |
Arthrotomy |
Acute synovitis |
Cured |
|
9 |
Knee septic arthritis |
Arthrotomy |
Chronic osteomyelitis |
Cured |
|
10 |
Femur osteomyelitis |
Nonsurgical |
Not done |
Cured |
|
11 |
Tibia osteomyelitis |
Nonsurgical |
Not done |
Died |
|
12 |
Multiple abscess in femur |
Nonsurgical |
Not done |
Cured |
|
13 |
Ankle septic arthritis |
Nonsurgical |
Not done |
Cured |
|
14 |
Sternum abscess |
Nonsurgical |
Not done |
Cured |
|
15 |
Soft tissue abscess |
Nonsurgical |
Not done |
Died |
|
16 |
Humerus osteomyelitis |
Decompression |
Acute synovitis |
Cured |
|
17 |
Shoulder septic arthritis |
Arthrotomy |
Acute synovitis |
Cured |
|
18 |
Femur osteomyelitis |
Decompression |
Acute synovitis |
Cured |

Twelve patients (66%) required surgical management. The procedures performed included arthrotomy for septic arthritis, decompression for osteomyelitis (
Figs. 1 and
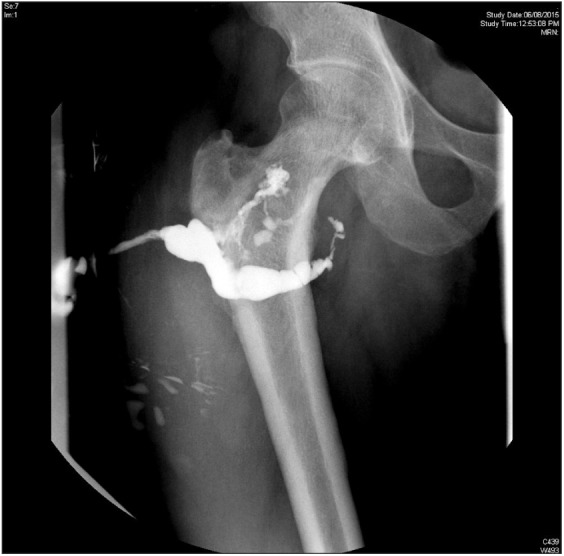
2) and drainage and debridement for soft tissue abscesses. Three patients required local antibiotic delivery with antibiotic loaded polymethylmethacrylate beads as an adjunct to the primary surgical procedure. Two patients had a pathological fracture of the femur during the course of antibiotic treatment and required internal fixation (
Fig. 3) and one patient had quadricepsplasty for postoperative knee stiffness. In all, 9 out of 18 patients required more than 1 surgical procedure. There were 2 patients (33.3%) who died among those who had nonsurgical management as compared to none in the cohort who had surgical management (significant at 10% level of significance;
p = 0.098) (
Table 3). All patients had a full course of antibiotic therapy with intravenous ceftazidime followed by oral doxycycline and cotrimoxazole. Two patients who died during the course of treatment due to septicemia had multisystem involvement with positive blood cultures. The rest were followed up for a minimum of 1 year (range, 1 to 5 years). There was no evidence of disease recurrence noted in any of the patients. There were no long-term clinically significant sequelae.
 | Fig. 1Sinusogram showing a foci of osteomyelitis in the proximal femur.
|
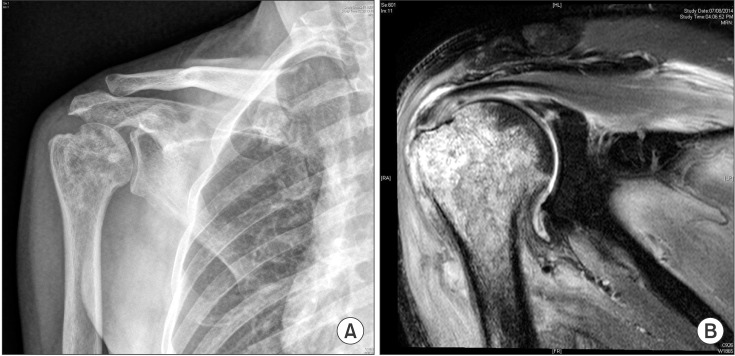 | Fig. 2Osteomyelitis of the proximal humerus on plain radiography (A) and magnetic resonance imaging (B).
|
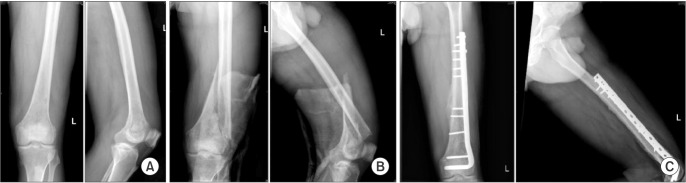 | Fig. 3Serial radiographs of a patient with distal femur osteomyelitis. (A) Post-debridement radiographs. (B) The patient developed a fracture of the distal femur 2 months after debridement. (C) The fracture was fixed and showed uncomplicated union.
|
Table 3
Comparison of Final Outcome between Nonsurgical and Surgical Treatment Groups
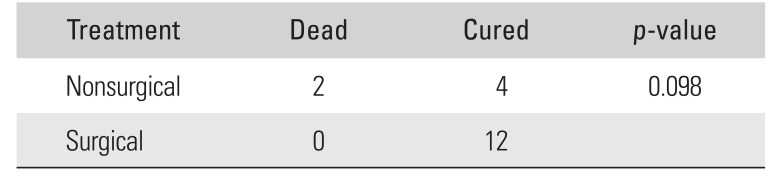
|
Treatment |
Dead |
Cured |
p-value |
|
Nonsurgical |
2 |
4 |
0.098 |
|
Surgical |
0 |
12 |
|

Go to :

DISCUSSION
The present series describing musculoskeletal involvement in melioidosis is the largest such study from what may be considered as a recently recognized ‘endemic’ region. Of importance in the study are the changing demographics of the affected population, the prevalence of musculoskeletal involvement in patients with septicemic and multisystem involvement and encouraging outcomes with surgical intervention.
The traditional at risk population for melioidosis were those exposed to this soilborne pathogen, such as occupations involving farming, especially in regions of Southeast Asia and Northern Australia.
10) With improving diagnostic modalities, melioidosis is being increasingly recognized around the world, particularly in the tropics. Thirteen of our patients were sedentary office workers who do not identify with the traditional at risk population, suggesting that the disease is maybe underdiagnosed in the urban community.
The incidence of musculoskeletal involvement in melioidosis has been reported to range from 4% to 12%, usually as a part of multisystem involvement.
7891112) In our series, the prevalence of musculoskeletal involvement was 14% with predominant involvement of the lower limbs. The nonspecific clinical, radiological, and histopathological features were considered reasons for delay in diagnosis. The diagnostic delay in this series ranged from 1 month to 9 months (average, 3.2 months). Thirty-eight percent of patients in this series had prior empirical antitubercular therapy initiated by the primary treating physician. In India, with tuberculosis being very common, most patients have empiric treatment for tuberculosis especially when definitive diagnosis has not been established in a prolonged febrile illness. Association with diabetes mellitus has been reported in 23% to 60% of patient with this infection.
910) In this series, 61% of patients were diabetic.
Numerous reports have stressed upon the histopathological feature of melioidosis as chronic granulomatous inflammation practically indistinguishable from tuberculosis.
56) However, in this series only 1 patient had such a histopathological feature. The majority of patients in this series had histopathology suggestive of inflammatory granulation tissue, without evidence of granuloma formation, akin to pyogenic infections. Hence, it must be emphasised that though the granulomatous histologic appearance of melioidosis is important, it is not entirely diagnostic.
Musculoskeletal involvement of melioidosis may present septic arthritis, osteomyelitis or soft tissue abscesses. Pathological fracture as a clinical presentation in bone weakened by the disease process was seen in 2 of our patients.
Twelve patients in this series underwent surgical management, including arthrotomy, debridement, decompression, external fixation, antibiotic bead placement and internal fixation. The remaining patients had diagnosis confirmed by a biopsy and were subsequently treated with antibiotics. The encouraging results of operative intervention in our study concur with those reported in literature. In a study by Pandey et al.,
12) surgical management of skeletal melioidosis led to good results with no recurrences in 4 of 5 patients, which was similar to our findings in terms of cure rate. The use of antibiotic impregnated composite in the management of melioidotic osteomyelitis too has been described in studies by Ng et al.
13) and Subhadrabandhu et al.
14)
All cases of melioidosis should be treated with initial intensive therapy (at least 2 weeks of intravenous therapy) followed by oral eradication therapy for a minimum of 6 to 12 months. The drug of choice for the intensive phase is ceftazidime.
15) Carbepenems are also recommended and cotrimaoxazole is given during the intensive phase. In our case series the patients received a combination of cotrimoxazole and doxycycline during the maintenance phase, which is the standard of care in our centre during the period when the data was collected. However, current evidence suggests that cotrimoxazole may be given as a single agent as well.
16)
Septicemic melioidosis can cause multisystem failure. Two of 9 patients with positive blood cultures expired despite being started on appropriate antimicrobial therapy after diagnosis. None of these patients were given granulocyte-colony stimulating factor as no benefit has been shown in randomised control trials.
17) The survival rates in this study are similar to those documented in other observational studies thus emphasizing that the septicemic form, if not diagnosed early, can be lethal.
18) In this study 16 surviving patients had complete recovery at their last follow-up.
Early diagnosis of emerging infections such as melioidosis is the need of the hour. A high index of suspicion whilst treating patients with unexplained pyrexia is vital. Obtaining timely tissue cultures and using simple, reproducible diagnostic modalities such as coagglutination tests for antibodies aid in early identification of the infection. With diagnostic facilities not being universally available, it is prudent to refer undiagnosed infections to tertiary care centers at the earliest.
Despite being the largest series of patients with musculoskeletal melioidosis from a nonendemic region, the lack of more patients and longer follow-up period are limitations of the study.
In conclusion, the present study stresses the significance of obtaining an early microbiological diagnosis as demographics and patterns of musculoskeletal involvement are varied. Adequate cure without recurrence can be obtained with appropriate surgical intervention complemented by targeted antibiotic therapy.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download