Abstract
Background
Venous thromboembolism (VTE) is a relatively common and potentially life threatening complication after major hip surgery. There are two main types of prophylaxis: chemical and mechanical. Chemical prophylaxis is very effective but causes bleeding complications in surgical wounds and remote organs. On the other hand, mechanical methods are free of hemorrhagic complications but are less effective. We hypothesized that mechanical prophylaxis is effective enough for Asians in whom VTE occurs less frequently. This study evaluated the effect of intermittent pneumatic compression (IPC) in the prevention of VTE after major hip surgery.
Methods
Incidences of symptomatic VTE after primary total hip arthroplasty with and without application of IPC were compared. A total of 379 patients were included in the final analysis. The IPC group included 233 patients (106 men and 127 women) with a mean age of 54 years. The control group included 146 patients (80 men and 66 women) with a mean age of 53 years. All patients took low-dose aspirin for 6 weeks after surgery. IPC was applied to both legs just after surgery and maintained all day until discharge. When a symptom or a sign suspicious of VTE, such as swelling or redness of the foot and ankle, Homans' sign, and dyspnea was detected, computed tomography (CT) angiogram or duplex ultrasonogram was performed.
Results
Until 3 months after surgery, symptomatic VTE occurred in three patients in the IPC group and in 6 patients in the control group. The incidence of VTE was much lower in the IPC group (1.3%) than in the control group (4.1%), but the difference was not statistically significant. Complications associated with the application of IPC were not detected in any patient. Patients affected by VTE were older and hospitalized longer than the unaffected patients.
Venous thromboembolism (VTE) comprising deep vein thrombosis (DVT) and pulmonary embolism (PE) is one of the major complications after major hip surgery.12) PE occurs much less frequently than DVT, but it is a major cause of death after major hip surgery.3) The injury of soft tissue including the vessel wall during operation and patient immobilization after a hip surgery are inevitable and known to increase the risk of VTE.4) The incidence of VTE in Western countries after total hip arthroplasty (THA) without any prophylaxis is reported to range from 45% to 57%.5)
Because of the high incidence, rare but possible fatality, and subsequent post-thrombotic syndrome, active prophylaxis of VTE is strongly recommended in the perioperative period.67) There are two main categories of prophylaxis: chemical and mechanical. Chemical prophylaxis uses anticoagulants. Newly developed anticoagulants such as low molecular heparin and factor Xa inhibitors (pentasaccharide) are widely used instead of heparin and wafarin.68) Anticoagulants are very effective in VTE prophylaxis but have the risk of bleeding in the operation site and other sites such as intracranial and gastrointestinal sites.46)
For mechanical prophylaxis, graduated compression stockings and intermittent pneumatic compression (IPC) devices are used.9) Mechanical methods have no risk of unexpected bleeding and can be applied to the contralateral leg during operation. After surgery, these methods can be applied any time regardless of drain status. However, there have been debates on the efficacy of mechanical methods in VTE prevention. The American College of Chest Physicians (ACCP) recommended mechanical methods alone primarily in patients at high risk of bleeding until the 8th edition of its guidelines.8)
It has been considered that VTE occurs less frequently in Asians and surgeons have not recognized the necessity of perioperative prophylaxis.11011) However, in 2005, two multicenter studies conducted in Asian countries reported that the incidence of postoperative VTE was not low in Asians, and it was consistent with that in Western countries.512) The two reports prompted interest in perioperative VTE and its prophylaxis in Asian surgeons, some of whom started to insist on aggressive prophylaxis using anticoagulants. Resultantly, the use of chemoprophylaxis has increased. However, its use for Asian patients who have a relatively low VTE incidence has been controversial because of the bleeding risk.13) Therefore, mechanical methods using intermittent pneumatic pumps have been gaining popularity, and variable results on their effectiveness have been reported in Asian countries.14151617)
We have routinely used low-dose aspirin (100 mg qd) in hip surgery patients. Since March 2010, this monotherapy has been augmented by IPC in some patients. Since December 2010, both methods have been applied to all patients after major hip surgery unless contraindicated. In this study, we evaluated the prophylactic effect of IPC by comparing the incidences of symptomatic VTE before and after the use of IPC and analyzed the characteristics of the patients who were affected by VTE.
This study was approved by the Institutional Review Board of the Seoul National University Hospital (No. H-1403-050-565). The incidences of VTE of the IPC group and control group were compared retrospectively. The IPC group included consecutive patients who underwent primary THA between January 2011 and December 2012. In these patients, IPC was used in addition to low-dose aspirin. The control group included consecutive patients who underwent THA between March 2008 and February 2010. In these patients, only low-dose aspirin was used. For both groups, patients were excluded if they were younger than 17 years, taking anticoagulant stronger than aspirin for any reason, had history of previous VTE, could not take aspirin for any reason, and were followed up for less than 3 months after surgery.
THA was performed by one of the two senior surgeons (HJK and JJY) usually under spinal anesthesia. Administration of low-dose aspirin (100 mg po qd) started after removal of drainage at postoperative 2 or 3 days and continued for 6 weeks. IPC was started after operation and continued until discharge using a mechanical pump (Kendall SCD EXPRESS; Covidien, Mansfield, MA, USA) (Fig. 1). Both calves were compressed all day long except when patients were out of bed. All patients started active leg muscle contraction exercise when recovered from anesthesia and started crutch walking as soon as possible. Patients were discharged when they could walk with a walker or crutches. The average period of hospitalization was 10.1 days in the IPC group and 11.9 days in the control group.
Postoperatively, patients were observed closely for VTE. When any symptom or sign suspicious of VTE was observed, computed tomography (CT) angiography was performed to detect DVT and PE. Suspicious symptoms and signs included swelling or redness in the foot and ankle, pain or tenderness in the leg, Homans' sign, and dyspnea. If CT angiography was contraindicated because of the patient's condition, such as renal insufficiency, duplex ultrasonography was performed. Patients were followed up at postoperative 6 weeks, 3 months, 9 months, and annually thereafter. At every follow-up, CT angiography or ultrasonography was performed if VTE was suspected.
The VTE incidences until 3 months after THA were compared between the two groups and risk factors associated with the development of VTE were determined.1819) For statistical analyses, Fisher exact test and independent t-test were used depending on data, with statistical significance set at p < 0.05.
There was no notable difference between the two groups in sex ratio, age distribution, body mass index (BMI), comorbidity index, underlying disease for THA, method of anesthesia, and operation time (Tables 1,2,3). There was a significant difference in the hospitalization period: it was longer in the control group (11.9 ± 5.3 days) than in the IPC group (10.1 ± 2.8 days) (p < 0.001).
In the IPC group, there was no case of complication related with IPC application, such as skin bullae or ulcer and compartment syndrome. Most patients had some intermissions in applying IPC, but patient's compliance was generally good.
In the IPC group, symptomatic VTE developed in three cases (distal DVT with PE, proximal DVT with PE, and proximal DVT only). In the control group, symptomatic VTE developed in six cases (three cases of distal DVT, one case of proximal DVT, and two cases of proximal and distal DVT). There was no case combined with PE (Table 4). VTE was detected at an average of 39 days (range, 6 to 78 days) after surgery: during hospitalization in 3 cases and after discharge in 6 cases. All DVT developed in the operated leg except in one case in which both legs were affected (Table 5). The incidence of VTE was 1.3% in the IPC group and 4.1% in the control group, but the difference was not statistically significant (p = 0.093). The incidence of proximal DVT was lower in the IPC group and the incidence of PE was lower in the control group, but the differences were not statistically significant. The incidence of distal DVT was significantly lower in the IPC group (0.4%) than in the control group (4.1%) (p = 0.015) (Table 5).
Patients affected by VTE were older and hospitalized longer with statistical significance (p = 0.030, p = 0.012 respectively). However, sex, BMI, comorbidity index, and operation time were not associated with the development of VTE (Table 6).
Among the patients affected by VTE, 3 cases were treated by anticoagulant for 3–13 months, 1 case was treated by thrombectomy and anticoagulant for 3 years, and the other cases were simply observed.
In this study, the incidence of VTE in the IPC group was less than 30% of that in the control group. This remarkable difference suggests the effectiveness of IPC in prevention of VTE even though the difference was not significant statistically. Foot or low leg pneumatic pump has been used for the prevention of VTE for 30 years and there have been many reports of its effectiveness.202122) However, the ACCP guidelines had not recommended single use of IPC except for patients with high bleeding risk because of the absence of well-controlled prospective studies on IPC.8) In 2012, the ACCP included IPC as a single modality for the prevention of VTE after major orthopaedic surgery.23) Then effectiveness of this strategy has been confirmed in meta-analyses of previous reports and prospective studies.222425) There have been a few reports on preventive effect of IPC in Asians. Sugano et al.14) and Yokote et al.16) reported its effectiveness in Japanese people, Mehta et al.15) in Singapore people, and Jo et al.17) in Korean people.
Patient compliance with IPC is known to be very good.2627) Some complications of IPC have been reported; most are minor and include skin bullae and itching from the compression pad. One case report described compartment syndrome, but serious complications are rare.28) In this study, no patient stopped IPC because of discomfort and no IPC-related complications occurred.
In South Korea, the reported incidence of symptomatic VTE after THA ranges from 0% to 2.2%.129) The incidence of VTE in this study was high compared with that of previous studies, even though low-dose aspirin was administered. This study was not a prospective one, but CT angiography or ultrasonography was performed whenever suspicious symptoms and signs were detected, even if they were not eminent but vague. Of the 39 patients examined by CT angiography or ultrasonography, only a few patients had calf tenderness or Homans' sign. This might have resulted in the higher detection rate.
Many factors have been reported to be associated with the occurrence of VTE. These include history of previous VTE, cardiovascular disease, comorbidity index, obesity, old age, varicose vein, and prolonged bed rest.1819) In this study, only old age and long admission period were associated with the occurrence of VTE, whereas sex, obesity, operation time, and comorbidity were not. The number of VTE cases (n = 9) might have been too small for a risk factor analysis.
This study has some limitations. It is a retrospective study with a relatively small number of cases and only the patients with suspicious symptoms or signs were examined by CT angiography or ultrasonography. Therefore, the true incidence of VTE was unknown. However, consecutive patients who received constant perioperative management in a single institute were included in the analysis. Therefore, the limitations did not have a substantial effect on the evaluation of the effectiveness of IPC in the prevention of VTE.
In this study, the incidence of symptomatic VTE after THA was 1.2% in patients receiving IPC in addition to low-dose aspirin. For Western people, the incidence of symptomatic VTE after THA has been reduced to 1%–2.5% using chemoprophylaxis.630) Considering the bleeding complications of chemoprophylaxis, low-dose aspirin with IPC can be suggested as an effective and safe option to prevent postoperative VTE for Asians.
In conclusion, the results of this study suggest IPC might be an effective method to prevent postoperative VTE, although statistical significance was not observed.
Figures and Tables
 | Fig. 1Main body with accessories (A) and cuff (B) of the intermittent pneumatic pump used in this study. |
Table 1
Patient Demographics

Table 2
Etiologic Disease of Total Hip Arthroplasty

Table 3
The Comparison of Operation Related Factors

Table 4
Incidence of Venous Thromboembolism
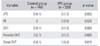
| Variable | Control group (n = 146) | IPC group (n = 233) | p-value |
|---|---|---|---|
| VTE | 6 (4.1) | 3 (1.3) | 0.093 |
| PE | 0 | 2 (0.9) | 0.525 |
| DVT | 6 (4.1) | 3 (1.3) | 0.093 |
| Proximal DVT | 3 (2.1) | 3 (1.2) | 0.680 |
| Distal DVT | 6 (4.1) | 1 (0.4) | 0.015 |
Table 5
Anatomical Sites of Thrombi in Venous Thromboembolism of Each Patient
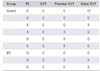
| Group | PE | DVT | Proximal DVT | Distal DVT |
|---|---|---|---|---|
| Control | X | O | O | O* |
| X | O | O | O | |
| X | O | O | X | |
| X | O | X | O | |
| X | O | X | O | |
| X | O | X | O | |
| IPC | O | O | O | O |
| O | O | O | X | |
| X | O | O | X |
Table 6
Risk Factors of VTE

ACKNOWLEDGEMENTS
This study was supported by a grant from the Seoul National University Hospital Research Fund (No. 06-03-0630).
References
1. Kim YH, Oh SH, Kim JS. Incidence and natural history of deep-vein thrombosis after total hip arthroplasty: a prospective and randomised clinical study. J Bone Joint Surg Br. 2003; 85(5):661–665.
2. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe: the number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007; 98(4):756–764.
3. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop Relat Res. 2009; 467(1):213–218.

4. Fisher WD. Impact of venous thromboembolism on clinical management and therapy after hip and knee arthroplasty. Can J Surg. 2011; 54(5):344–351.

5. Piovella F, Wang CJ, Lu H, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia: an epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005; 3(12):2664–2670.

6. Warwick D. New concepts in orthopaedic thromboprophylaxis. J Bone Joint Surg Br. 2004; 86(6):788–792.

7. Rabinovich A, Cohen JM, Kahn SR. Predictive value of markers of inflammation in the postthrombotic syndrome: a systematic review: inflammatory biomarkers and PTS. Thromb Res. 2015; 136(2):289–297.

8. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:6 Suppl. 381S–453S.
9. Morris RJ, Woodcock JP. Evidence-based compression: prevention of stasis and deep vein thrombosis. Ann Surg. 2004; 239(2):162–171.
10. Stein PD, Kayali F, Olson RE, Milford CE. Pulmonary thromboembolism in Asians/Pacific Islanders in the United States: analysis of data from the National Hospital Discharge Survey and the United States Bureau of the Census. Am J Med. 2004; 116(7):435–442.

11. White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005; 93(2):298–305.

12. Leizorovicz A, Turpie AG, Cohen AT, et al. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis: the SMART study. J Thromb Haemost. 2005; 3(1):28–34.

13. Kanchanabat B, Stapanavatr W, Meknavin S, Soorapanth C, Sumanasrethakul C, Kanchanasuttirak P. Systematic review and meta-analysis on the rate of postoperative venous thromboembolism in orthopaedic surgery in Asian patients without thromboprophylaxis. Br J Surg. 2011; 98(10):1356–1364.

14. Sugano N, Miki H, Nakamura N, Aihara M, Yamamoto K, Ohzono K. Clinical efficacy of mechanical thromboprophylaxis without anticoagulant drugs for elective hip surgery in an Asian population. J Arthroplasty. 2009; 24(8):1254–1257.

15. Mehta KV, Lee HC, Loh JS. Mechanical thromboprophylaxis for patients undergoing hip fracture surgery. J Orthop Surg (Hong Kong). 2010; 18(3):287–289.

16. Yokote R, Matsubara M, Hirasawa N, Hagio S, Ishii K, Takata C. Is routine chemical thromboprophylaxis after total hip replacement really necessary in a Japanese population? J Bone Joint Surg Br. 2011; 93(2):251–256.

17. Jo WL, Lee YK, Ha YC, Lee KM, Kang BJ, Koo KH. Preventing venous thromboembolism with use of intermittent pneumatic compression after total hip arthroplasty in Korean patients. J Korean Med Sci. 2016; 31(8):1319–1323.

18. Bagaria V, Modi N, Panghate A, Vaidya S. Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: results of a prospective study. Postgrad Med J. 2006; 82(964):136–139.

19. Memtsoudis SG, Besculides MC, Gaber L, Liu S, Gonzalez Della Valle A. Risk factors for pulmonary embolism after hip and knee arthroplasty: a population-based study. Int Orthop. 2009; 33(6):1739–1745.

20. Gallus A, Raman K, Darby T. Venous thrombosis after elective hip replacement: the influence of preventive intermittent calf compression and of surgical technique. Br J Surg. 1983; 70(1):17–19.

21. Lachiewicz PF, Soileau ES. Multimodal prophylaxis for THA with mechanical compression. Clin Orthop Relat Res. 2006; 453:225–230.

22. Pour AE, Keshavarzi NR, Purtill JJ, Sharkey PF, Parvizi J. Is venous foot pump effective in prevention of thromboembolic disease after joint arthroplasty: a meta-analysis. J Arthroplasty. 2013; 28(3):410–417.

23. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:2 Suppl. e278S–e325S.
24. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013; 128(9):1003–1020.

25. Colwell CW Jr, Froimson MI, Anseth SD, et al. A mobile compression device for thrombosis prevention in hip and knee arthroplasty. J Bone Joint Surg Am. 2014; 96(3):177–183.

26. Werbel GB, Shybut GT. Acute compartment syndrome caused by a malfunctioning pneumatic-compression boot: a case report. J Bone Joint Surg Am. 1986; 68(9):1445–1446.

27. McAsey CJ, Gargiulo JM, Parks NL, Hamilton WG. Patient satisfaction with mobile compression devices following total hip arthroplasty. Orthopedics. 2014; 37(8):e673–e677.

28. Won SH, Lee YK, Suh YS, Koo KH. Extensive bullous complication associated with intermittent pneumatic compression. Yonsei Med J. 2013; 54(3):801–802.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download