Abstract
Background
There are limited data available regarding the results of reverse shoulder arthroplasty (RSA) in patients with rheumatoid arthritis (RA). We performed a systematic review of the literature to investigate the radiological and clinical outcomes after RSA in patients with RA.
Methods
A literature search for publications between 1987 and 2014 was conducted by 2 independent reviewers using PubMed, Scopus, Embase, and Cochrane Central Register of Controlled Trials. Articles were retrieved by an electronic search using keywords and their combinations. Studies that met inclusion criteria were assessed for pertinent data.
Results
Seven studies including 123 shoulders met the inclusion criteria. The mean age of the patients was 67.9 years and the mean follow-up period was 46.6 months. The mean Constant score and American Shoulder and Elbow Surgeons (ASES) score increased from 18.6 and 27.5 preoperatively to 58.6 and 73.7, respectively, at the final follow-up evaluation. The mean active forward flexion, abduction, and external rotation increased from 57.2°, 50.4°, and 11.4° to 127.1°, 116.7°, and 26.4°, respectively. The incidence of scapular notching was 33.7%. Twenty-seven (22.0%) of 123 shoulders had one or more complications, 12 of which (44.4%) had intraoperative or postoperative fractures. Nine shoulders (7.3%) had one or more revision surgeries.
Conclusions
RSA in RA showed similar short- to mid-term results without higher complication rates as compared to RSA in cuff tear arthropathy. Although RSA can be considered a reliable treatment option in patients with RA, further large-scale studies are required to determine the long-term survival of the implant.
Shoulder involvement in patients with rheumatoid arthritis (RA) is typically associated with not only advanced arthritic destruction of the glenohumeral joint but also rotator cuff tears.12) Progressive upward migration of the humeral head is an inevitable long-term consequence of the disease, indicating progressive rotator cuff failure.13) Therefore, patients with RA ultimately develop a condition similar to cuff tear arthropathy.245)
Reverse shoulder arthroplasty (RSA), which was originally designed by Grammont and his associates67) in 1987, has been widely used in patients with cuff tear arthropathy and irreparable massive rotator cuff tears. Recently, the indications have expanded to include RA, acute fractures, fracture sequelae, and revision surgery. Numerous studies have reported that RSA produced good results in terms of pain relief, function, and satisfaction for patients with cuff tear arthropathy or irreparable massive rotator cuff tears.4589) However, studies describing the results of RSA in patients with RA have been rarely reported and included only small numbers of patients.261011121314) Therefore, the benefits of this procedure remain controversial. The objective of this study was to systematically review the published data on the radiological and clinical outcomes as well as the complications after RSA in patients with RA.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systemic reviews.15)
We searched the medical and scientific literature included in PubMed, Scopus, Embase, and the Cochrane Central Register of Controlled Trials published between 1987 and 2014. Articles were retrieved by an electronic search using keywords and their combinations. We employed a text search strategy using a search string of “reverse shoulder OR rheumatoid arthritis OR Grammont arthroplasty OR inflammatory arthritis” excluding all articles not pertaining to the shoulder. The electronic search for relevant articles was performed by 2 independent reviewers.
Inclusion criteria for studies in this systematic review were as follows: (1) published in English; (2) involving RSA, not including total shoulder arthroplasty (TSA) or hemiarthroplasty; (3) involving a mean follow-up of more than 24 months after surgery; and (4) reporting explicit outcome data, with at least one of the primary outcomes including a scoring system or range of motion (ROM). The exclusion criteria included any review or surgical technique article, biomechanical study, or case report study.
Abstracts with clearly or potentially relevant titles were reviewed for relevance to RSA and RA and considered appropriate for inclusion. If a title was clearly irrelevant, the publication was excluded. Additionally, the authors manually reviewed all references from all studies that met the inclusion criteria to generate a list of qualifying studies not identified by the electronic searches. We extracted and analyzed the study design, demographic variables, outcomes, and complications. The 2 authors (CHC and DHK) independently extracted the data, then conferred and compiled the data in order to resolve any discrepancies.
We initially obtained 354 unique articles using the aforementioned search criteria. Of those, 326 articles were excluded due to the lack of relevance as assessed by the article title or the study question. Subsequently, 9 articles were excluded based on the content of the associated abstracts. Then, the remaining 19 articles with an abstract deemed to have the potential to address the study question was selected for full text review, which resulted in the exclusion of 12 articles that fail to meet the inclusion criteria. This systematic review ultimately obtained 7 articles for analysis. References were manually searched for additional articles that fit the inclusion criteria, which were screened in the same systematic manner. No additional articles that fit the inclusion criteria were identified in this process. None of the included articles were written by the same author (Fig. 1), and all of the included studies were published between 2001 and 2012.
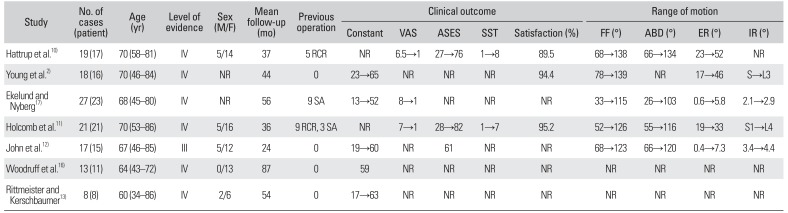
We extracted data on 123 shoulders (109 patients) from the 7 primary studies.2101112131617) Patient demographic information was pooled. Complete demographics regarding age, sex, and mean follow-up period were provided by almost all of the included studies. The mean age of the patients at the time of surgery was 67.9 years (range, 34 to 86 years), and the majority (78.2%) were female. The mean duration of follow-up was 46.6 months (range, 24 to 87 months). All 123 shoulders of 109 patients had major damage to the rotator cuff or destructive changes of the shoulder joint identified on preoperative imaging. Twenty six shoulders had undergone prior operations including rotator cuff repair in 14 shoulders and arthroplasty in 12 shoulders (Table 1).
The Delta III prosthesis (DePuy, Warsaw, IN, USA) was implanted in all cases in 3 studies.121316) An Aequalis Reverse Shoulder (Tornier, Amsterdam, The Netherlands) in one and a DJO prosthesis (DJO Surgical, Vista, CA, USA) in another study.211) Ekelund and Nyberg17) initially implanted the Delta III and subsequently the Delta Xtend. Hattrup et al.10) used implants from 4 different companies: 8 Delta III, 7 Trabecular Metal Reverse Shoulders (Zimmer, Warsaw, IN, USA), 2 Aequalis Reverse Shoulder, and 2 DJO prosthesis.
Almost all included studies reported significant improvements in all outcome measurements, and 1 study reported good clinical results at the final follow-up.16) The Constant score was used as the measure of functional outcome in five studies.112131617) The mean Constant score increased from 18.6 (range, 13 to 23) before surgery to 58.6 (range, 52 to 65) at the final follow-up evaluation. The American Shoulder and Elbow Surgeons (ASES) score was used in three studies.101112) The mean ASES score increased from 27.5 (range, 27 to 28) before surgery to 73.7 (range, 61 to 82) at the final follow-up evaluation. The Simple Shoulder Test (SST) score was used in 2 studies.1011) The mean SST score increased from 1 before surgery to 7.5 (range, 7 to 8) at the final follow-up evaluation. The visual analog scale (VAS) score was used to describe pain intensity in 3 studies. 101117) The mean VAS score improved from 7.3 (range, 6.5 to 8) before surgery to 1 at the final follow-up evaluation. Three studies reported a mean postoperative satisfaction rate of 93.0% (range, 89.5% to 94.4%).21011)
Data on active forward flexion and external rotation were reported in 5 studies and abduction and internal rotation in 4 studies.210111217) The mean forward flexion increased from 57.2° (range, 33° to 78°) before surgery to 127.1° (range, 115° to 139°) at the final follow-up evaluation. The mean abduction increased from 50.4° (range, 26° to 66°) before surgery to 116.7° (range, 103° to 134°) at the final follow-up evaluation. The mean external rotation increased from 11.4° (range, 0.4° to 23°) before surgery to 26.4° (range, 5.8° to 52°) at the final follow-up evaluation. There was no notable improvement in internal rotation after surgery.
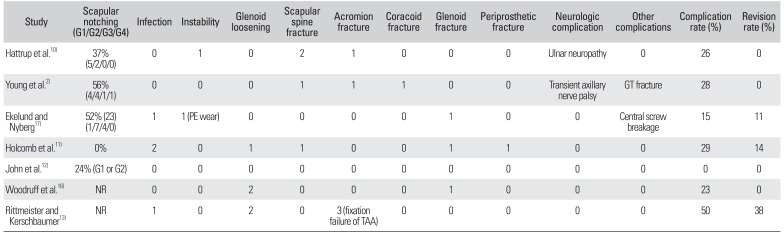
Scapular notching, one of the most common problems after RSA, was reported in 5 studies.210111217) Thirty three of 102 shoulders had scapular notching (mean incidence, 32.4%; range, 0 to 56%). According to the classification system developed by Sirveaux et al.,18) mild scapular notching (grade 1 or 2) was observed in 27 cases (81.8%), and a moderate-to-severe notching (grade 3 or 4) was observed in 6 cases (18.2%).
A complication was defined as any intraoperative or postoperative event that was likely to have a negative influence on the patient's outcome. Collectively, all 7 studies reported that 27 of 123 cases had one or more complications with an overall complication rate of 22.0% (range, 0 to 50%).2101112131617) The complications included 4 infections, 2 instances of instability or dislocation, 5 cases of glenoid loosening, 3 fixation failures of the acromion after the transacromial approach, 2 neurologic complications (ulnar nerve neuropathy and transient axillary nerve palsy), and 1 central screw breakage. Other complications related to intraoperative or postoperative fractures included 4 scapular spine fractures, 2 acromion fractures, 1 coracoid fracture, 3 glenoid fractures, 1 periprosthetic fracture, and 1 fracture of the greater tuberosity.
Nine cases underwent 12 revision surgeries due to infections, fixation failures of the acromion by the transacromial approach, glenoid loosening, instability attributable to polyethylene wear, periprosthetic fractures, or central screw breakage. The overall revision rate was 7.3% (range, 0 to 38%) (Table 2).
Although RSA has been used as a treatment option in patients with RA, a controversy regarding its benefits after surgery remains. Several studies emphasized that RSA in patients with RA had poorer clinical outcomes and higher complication and revision rates including intraoperative fractures, glenoid loosening, and infection than RAS in patients with other etiologies.192021) Guery et al.20) stated that extreme caution should be exercised regarding the use of RSA in patients with RA, as they had the highest percentage (25%) of implant revisions due to infection in the 8 rheumatoid cases in their series. However, they softened their position regarding the use of RSA in patients with RA in a further study.2) They posited that RSA should not be used for RA shoulders in the past; in a subsequent study, however, they found patients with RA experienced not only satisfactory pain relief but also statistically significant improvements in functional shoulder motion after RSA.2) Based on such results, the authors determined that RSA was a good procedure for patients with RA, and particularly for RA patients over 65 to 70 years of age.2) Recently, several studies on RSA in patients with RA showed similar short- to mid-term results without higher complication rates to those with cuff tear arthropathy.210111217) Therefore, RSA can be considered a reliable treatment option for patients with RA, resulting in significant pain relief and improvements in functional shoulder motion.
Aside from the paucity of published articles describing results after RSA in patients with RA, there is an additional confounding factor, the small number of patients per study. This prompted us to perform a systematic review of the literature on the radiological and clinical outcomes after RSA in patients with RA.
This systematic review revealed that RSA performed in patients with RA resulted in not only pain relief, but also significant improvements in functional shoulder motion. All 7 studies reported significant improvements in all outcome measurements, such as Constant score, VAS score, ASES score, SST score, Short Form (SF) score, and satisfaction rate.212131617) In 5 studies, the Constant score was used as an outcome measure, with the mean score increasing from 18.6 preoperatively to 58.6 at the final follow-up.212131617) In 3 studies, the ASES score increased from 27.5 before surgery to 73.7 after surgery.101112) Collectively, the results from three studies indicated an overall postoperative satisfaction rate of 92.4%.21011) As an outcome measure for quality of life, Woodruff et al.16) reported that 17 patients with RA of the shoulder showed satisfaction with their clinical outcomes as demonstrated by mean SF-12 scores of 33 on the physical component and 49 on the mental component. John et al.12) reported that the mental and physical component summary scores of the SF-36 were 54 and 30, respectively. The clinical results observed in this systematic review are comparable in large part to those described in studies on RSA in patients with cuff tear arthropathy.14921)
Regarding ROM, five studies provided data on active forward flexion and external rotation and 4 studies reported on abduction and internal rotation.210111217) Two studies did not mention any value for ROM improvement.1316) The mean forward flexion, abduction, and external rotation increased from 57.2°, 50.4°, and 11.4° before surgery to 127.1°, 116.7°, and 26.4° after surgery, respectively. However, there was no notable improvement in internal rotation after surgery. Ekelund and Nyberg17) reported on 27 RSAs performed in RA patients with a mean follow-up of 56 months, and found that active flexion improved from 33° to 115° and abduction from 26° to 103°. However, external rotation improved only from 0.6° to 5.8°, and no change in internal rotation was observed. Additionally, Young et al.2) reported an improvement in active shoulder flexion from an average of 77° before surgery to 139° after surgery, with a 94% patient satisfaction rate. They also found that patients with a normal teres minor muscle experienced better clinical outcomes than patients with teres minor muscle atrophy. Holcomb et al.11) also found that postoperative shoulder forward flexion improved reliably from 52° to 125°, with an 86% success rate when patients were followed up after a minimum of 2 years. These functional results are comparable to those observed in studies of RSA in patients with cuff tear arthropathy or irreparable massive rotator cuff tear.
Scapular notching is the most common problem after RSA, with an incidence of 0 to 96%.10162223242526) In this review, the mean incidence of scapular notching was 33.7% (range, 0 to 56%) at a mean follow-up of 46.6 months, which is consistent with those reported in studies of RSA in patients with cuff tear arthropathy or irreparable massive rotator cuff tear. Although most scapular notchings (81.8%) were regarded as mild (grade 1 or 2), longer-term follow-up studies will be required in order to determine the impact of scapular notching on clinical outcomes including the survival rates of the implants for patients with RA that undergo RSA.
According to the systematic review by Zumstein et al.,21) the overall complication rate was 24% for RSA in patients with all indications including cuff tear arthropathy, irreparable massive rotator cuff tear, osteoarthritis, acute fracture, fracture sequelae, RA, revision of a previous hemiarthroplasty or TSA, and tumor. The most common complication was instability (4.7%), followed by intraoperative or postoperative fractures (4.6%), infection (3.8%), glenoid loosening (3.5%), and acromion or scapular spine fractures (1.5%). The revision rate was 10.1%. In our systematic review of RSA in patients with RA, all 7 studies reported a collective overall complication rate of 20.4% (range, 0 to 38%). The overall revision rate was 7.3% (range, 0 to 38%). Therefore, the overall complication and revision rates were similar to those reported in other studies of RSA.
Several studies reported that there appeared to be a higher infection rate after RSA in patients with RA.111320) Guery et al.20) reported that 2 of 8 patients with RA (25%) developed postoperative infection. On the other hand, Ekelund and Nyberg17) reported that RA patients had a low risk for infection despite the immunocompromised state. Morris et al.27) reported that RA was not a risk factor for infection when controlling for other variables, such as diabetes, smoking, and prior failed arthroplasty. According to the systematic reviews, the overall incidence of postoperative infection following RSA for all indications was 3.8% with ranges from approximately 1% to 12%.421) In our systematic review, the infection rate following RSA in patients with RA was 3.3%, which was not considered to be high as compared to that observed in patients with cuff tear arthropathy in previous studies.
In 2001, Rittmeister and Kerschbaumer13) reported that 38% of RSA shoulders required surgical revisions due to aseptic loosening, infections, or fixation failures after utilizing a sabre-cut approach and acromion osteotomy. They emphasized that glenoid loosening remained a serious complication, and that transacromial approaches were complicated by failures of acromial fixation. However, they also found RSA results to be encouraging with respect to restoration of stability and satisfactory function in cuff deficient shoulders with RA. According to a study conducted by Woodruff et al.,16) radiographic analysis revealed evidence of radiolucency around the humeral component in all 17 cases, and around the glenoid component in 5 cases despite good clinical outcomes at a mean 87 months of follow-up evaluation. However, 3 recent studies have reported no glenoid or humeral loosening in a series of patients with RA who underwent RSA at short- to mid-term follow-up evaluations. Taken together, these results support the need for long-term follow-up studies to evaluate glenoid or humeral radiolucency and loosening.
One particular area of concern for RSA in patients with RA is intraoperative or postoperative fractures. Young et al.2) reported that fractures involving the acromion, acromial spine, coracoids, or greater tuberosity were observed either intraoperatively or postoperatively in 4 of the 18 shoulders (22.2%) in their study. The majority were intraoperative or postoperative fractures related to the increased bony fragility of the RA patients.2) Therefore, they emphasized that surgeons should be aware of the risk of intraoperative and postoperative fractures in patients with RA.
There is good evidence from the more recent literature that complication rates after RSA in patients with RA are improving. However, extreme care must be taken to prevent intraoperative and postoperative complications, such as infections, fractures, and component loosening.
Recently, 2 articles have been published on similar topics.2829) One was based on the only 5 studies that met their inclusion criteria.28) The other included 7 studies for critical appraisal and data extraction as in our study. However, there were some differences in the method to analyze the results. For example, Gee et al.29) included four studies in their analysis of shoulder range of motion; however, we found out that it was reported in detail in the 5 studies. In addition, we included 2 more patients for analysis of complications.1329)
This study has several limitations. First, only 7 studies including a total of 123 shoulders met our inclusion criteria for systematic review due to the paucity of published articles. Second, the mean follow-up period was 48.3 months. This may have been too short of a duration to detect clinically significant endpoints and to assess survivorship of the implants. Third, several studies did not provide complete data with regard to satisfaction rates, ROM, problems, and complications.
The main contribution of this study is that it provides the pertinent data including clinical outcomes, radiological results, problems, and complications after RSA in patents with RA through a systematic review.
In summary, RSA in patients with RA showed similar short- to mid-term results without higher complication rates as compared to those observed in cuff tear arthropathy or irreparable massive rotator cuff tears. Although RSA can be considered a reliable treatment option for patients with RA due to significant pain relief and improvements in functional shoulder motion, additional large-scale studies are required in order to determine the long-term survival rates of the implants.
References
1. Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013; 5(4):243–255. PMID: 24340143.

2. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011; 93(20):1915–1923. PMID: 22012529.

3. Lehtinen JT, Belt EA, Lyback CO, et al. Subacromial space in the rheumatoid shoulder: a radiographic 15-year follow-up study of 148 shoulders. J Shoulder Elbow Surg. 2000; 9(3):183–187. PMID: 10888161.

4. Jarrett CD, Brown BT, Schmidt CC. Reverse shoulder arthroplasty. Orthop Clin North Am. 2013; 44(3):389–408. PMID: 23827841.

5. Khan WS, Longo UG, Ahrens PM, Denaro V, Maffulli N. A systematic review of the reverse shoulder replacement in rotator cuff arthropathy, rotator cuff tears, and rheumatoid arthritis. Sports Med Arthrosc. 2011; 19(4):366–379. PMID: 22089287.

6. Grammont P, Trouilloud P, Laffay JP, Deries X. Etude et realisation d'une nouvelle prothese d'epaule. Rhumatologie. 1987; 39(10):407–418.
7. Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993; 16(1):65–68. PMID: 8421661.

8. Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013; 95(20):1877–1883. PMID: 24132362.
9. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012; 94(5):577–583. PMID: 22529074.
10. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012; 37(9):1888–1894. PMID: 22749484.

11. Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010; 19(7):1076–1084. PMID: 20363159.

12. John M, Pap G, Angst F, et al. Short-term results after reversed shoulder arthroplasty (Delta III) in patients with rheumatoid arthritis and irreparable rotator cuff tear. Int Orthop. 2010; 34(1):71–77. PMID: 19221749.

13. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001; 10(1):17–22. PMID: 11182731.

14. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005; 87(7):1476–1486. PMID: 15995114.

15. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151(4):264–269. PMID: 19622511.

16. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003; 27(1):7–10. PMID: 12582801.

17. Ekelund A, Nyberg R. Can reverse shoulder arthroplasty be used with few complications in rheumatoid arthritis? Clin Orthop Relat Res. 2011; 469(9):2483–2488. PMID: 21069486.

18. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004; 86(3):388–395. PMID: 15125127.
19. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012; 98(4 Suppl):S41–S47. PMID: 22583895.
20. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006; 88(8):1742–1747. PMID: 16882896.
21. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011; 20(1):146–157. PMID: 21134666.

22. Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg. 2014; 23(11):1662–1668. PMID: 24881833.
23. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006; 15(5):527–540. PMID: 16979046.

24. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005; 14(1 Suppl S):147S–161S. PMID: 15726075.

25. Levigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011; 469(9):2512–2520. PMID: 21116754.
26. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007; 89(3):588–600. PMID: 17332108.

27. Morris BJ, O'Connor DP, Torres D, Elkousy HA, Gartsman GM, Edwards TB. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015; 24(2):161–166. PMID: 25168350.

28. Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016; 40(5):965–973. PMID: 26202019.

29. Gee EC, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: a systematic review. Open Orthop J. 2015; 9:237–245. PMID: 26448802.

Fig. 1
Flow diagram of the systematic review process. RSA: reverse shoulder arthroplasty, RA: rheumatoid arthritis.
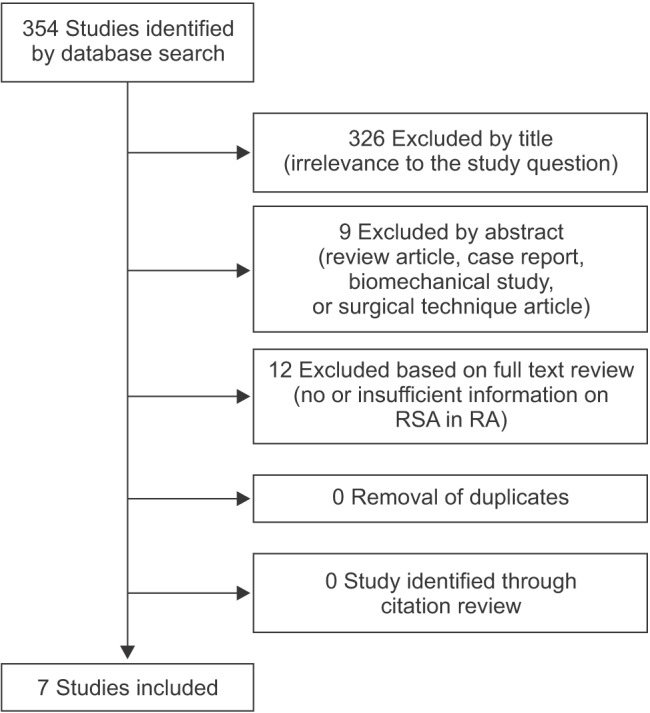
Table 1
Summary of Published Clinical Results of Reverse Total Shoulder Arthroplasty for Rheumatoid Arthritis
| Study | No. of cases (patient) | Age (yr) | Level of evidence | Sex (M/F) | Mean follow-up (mo) | Previous operation | Clinical outcome | Range of motion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constant | VAS | ASES | SST | Satisfaction (%) | FF (°) | ABD (°) | ER (°) | IR (°) | |||||||
| Hattrup et al.10) | 19 (17) | 70 (58–81) | IV | 5/14 | 37 | 5 RCR | NR | 6.5→1 | 27→76 | 1→8 | 89.5 | 68→138 | 66→134 | 23→52 | NR |
| Young et al.2) | 18 (16) | 70 (46–84) | IV | NR | 44 | 0 | 23→65 | NR | NR | NR | 94.4 | 78→139 | NR | 17→46 | S→L3 |
| Ekelund and Nyberg17) | 27 (23) | 68 (45–80) | IV | NR | 56 | 9 SA | 13→52 | 8→1 | NR | NR | NR | 33→115 | 26→103 | 0.6→5.8 | 2.1→2.9 |
| Holcomb et al.11) | 21 (21) | 70 (53–86) | IV | 5/16 | 36 | 9 RCR, 3 SA | NR | 7→1 | 28→82 | 1→7 | 95.2 | 52→126 | 55→116 | 19→33 | S1→L4 |
| John et al.12) | 17 (15) | 67 (46–85) | III | 5/12 | 24 | 0 | 19→60 | NR | 61 | NR | NR | 68→123 | 66→120 | 0.4→7.3 | 3.4→4.4 |
| Woodruff et al.16) | 13 (11) | 64 (43–72) | IV | 0/13 | 87 | 0 | 59 | NR | NR | NR | NR | NR | NR | NR | NR |
| Rittmeister and Kerschbaumer13) | 8 (8) | 60 (34–86) | IV | 2/6 | 54 | 0 | 17→63 | NR | NR | NR | NR | NR | NR | NR | NR |
Table 2
Summary of Reported Complications of Reverse Total Shoulder Arthroplasty for Rheumatoid Arthritis
| Study | Scapular notching (G1/G2/G3/G4) | Infection | Instability | Glenoid loosening | Scapular spine fracture | Acromion fracture | Coracoid fracture | Glenoid fracture | Periprosthetic fracture | Neurologic complication | Other complications | Complication rate (%) | Revision rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hattrup et al.10) | 37% (5/2/0/0) | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | Ulnar neuropathy | 0 | 26 | 0 |
| Young et al.2) | 56% (4/4/1/1) | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | Transient axillary nerve palsy | GT fracture | 28 | 0 |
| Ekelund and Nyberg17) | 52% (23) (1/7/4/0) | 1 | 1 (PE wear) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | Central screw breakage | 15 | 11 |
| Holcomb et al.11) | 0% | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 29 | 14 |
| John et al.12) | 24% (G1 or G2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Woodruff et al.16) | NR | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 23 | 0 |
| Rittmeister and Kerschbaumer13) | NR | 1 | 0 | 2 | 0 | 3 (fixation failure of TAA) | 0 | 0 | 0 | 0 | 0 | 50 | 38 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download