Abstract
Background
The pericytes in the blood vessel wall have recently been identified to be important in regulating vascular formation, stabilization, remodeling, and function. We isolated and identified pericyte-like platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells from the stromal vascular fraction (SVF) of adipose tissue from critical limb ischemia (CLI) patients and investigated their potential as a reliable source of stem cells for cell-based therapy.
Methods
De-identified subcutaneous fat tissues were harvested after amputation in CLI patients. Freshly isolated SVF cells and culture-expanded adipose-derived stem cells (ADSCs) were quantified using flow cytometry. A matrigel tube formation assay and multi-lineage differentiation were performed to assess pericytic and mesenchymal stem cell (MSC)-like characteristics of PDGFRβ+ ADSCs.
Results
PDGFRβ+ cells were located in the pericytic area of various sizes of blood vessels and coexpressed mesenchymal stem cell markers. PDGFRβ+ cells in freshly isolated SVF cells expressed a higher level of stem cell markers (CD34 and CXCR4) and mesenchymal markers (CD13, CD44, CD54, and CD90) than PDGFRβ– cells. In vitro expansion of PDGFRβ+ cells resulted in enrichment of the perivascular mesenchymal stem-like (PDGFRβ+/CD90+/CD45–/CD31–) cell fractions. The Matrigel tube formation assay revealed that PDGFRβ+ cells were located in the peritubular area.
Conclusions
PDGFRβ+ ADSCs cells demonstrated a good multilineage differentiation potential. Pericyte-like PDGFRβ+ cells from the SVF of adipose tissue from CLI patients had MSC-like characteristics and could be amplified by in vitro culture with preservation of their cell characteristics. We believe PDGFRβ+ cells in the SVF of adipose tissue can be used as a reliable source of stem cells even in CLI patients.
Chronic critical limb ischemia (CLI) is defined as the end stage of lower limb ischemia due to peripheral arterial disease (PAD). The prognosis of CLI patients is poor with an 1-year major amputation rate of 30%.12) Moreover, although revascularization of the ischemic limb with surgical bypass or endovascular approaches may be helpful for limb salvage, revascularization procedures may not be possible in the severe stage of CLI. Therefore, we suggest CLI be one of the main targets for cell therapy as stem cells and progenitor cells are believed to have an important function in tissue regeneration after damage.
On the basis of numerous successful animal experiments, cell therapies for PAD using bone marrow-derived mononuclear cells (BM-MNCs),34567) peripheral blood-derived mononuclear cells (PB-MNCs),89) and adipose-derived stem cells (ADSCs)110) have been reported to be promising, though more evidence needs to be accumulated before being used as a standard modality of treatment.
Among the various sources of stem or progenitor cells, adipose tissue represents an attractive source of autologous adult stem cells used in regenerative therapy owing to its abundance, surgical accessibility, and high content of multipotent mesenchymal stem cell (MSC)-like cells.11) It has been reported that the adipose tissue yields far more colony-forming units than the bone marrow when the weight of the tissues used is the same.12) However, knowledge of the cellular origin of culture-expanded mesenchymal-like ADSCs remains elusive because of the complex organization of stromal cells surrounding the small blood vessels.13)
Recent studies have suggested that the perivascular niche may be an important reservoir of MSCs in multiple developed organs.1415) While it is evident that not all perivascular cells are MSCs, the majority of MSCs are believed to reside in the perivascular area.16) Although there are no specific cell surface markers for perivascular cells or pericytes that regulate vessel stability and vascular survival, platelet-derived growth factor receptor beta (PDGFRβ) is thought to be a major cell surface marker defining the pericytes.1718)
We hypothesized that PDGFRβ-positive (PDGFRβ+) perivascular cells of the white adipose tissue from CLI patients would have characteristics of the MSCs and would be a good source for autologous cell transplantation as a treatment for CLI.
The purposes of our study were (1) to identify PDGFRβ+ perivascular cells in the white adipose tissue from CLI patients; (2) to isolate and characterize PDGFRβ+ cells from the stromal vascular fraction (SVF) of patient's adipose tissue in vitro; and (3) to investigate the differentiation potential of PDGFRβ+ cells as a reliable source of MSCs that can be used for the treatment of CLI.
This study was approved by Seoul National University Hospital Institutional Review Board (No. 1103-035-354) and informed consent was obtained from all patients. Among the 28 patients who had undergone lower limb amputation surgery (below knee amputation or above knee amputation) due to CLI from March 2010 to September 2011, 10 specimens from 10 patients were included in this study. The other 18 patients declined to participate and were excluded. Subcutaneous adipose tissue (1–3 g) was obtained aseptically from each amputated specimen and transported to our laboratory in aseptic bottles with phosphate buffered saline (PBS). Medical records were not profiled in accordance with IRB instructions as they could be used for the identification of tissue donors. Samples from three patients were allocated to immunofluorescence staining of fresh fat tissue and fluorescence-activated cell sorter (FACS) analysis of fresh SVF and remaining 7 samples were used after expansion to obtain enough amount of cells for extensive FACS analysis (triple samples for each analysis).
The SVF of adipose tissue was isolated as described in previous studies.192021) Fresh fat tissues were washed at least three times with PBS to remove blood, and then digested at 37℃ for 1 hour with 0.075% collagenase type 1 (Sigma-Aldrich, St. Louis, MO, USA) in Dulbecco's Modified Eagle Medium (DMEM; Gibco, New York, NY, USA). Mature adipocyte fractions were separated from stromal fractions by centrifuging at 1,200 × g for 10 minutes. The remained fractions were treated with red blood cell lysis buffer for 10 minutes at room temperature (RT) and then filtered through 100-µm nylon mesh to exclude remaining erythrocyte debris, and then centrifuged at 1,200 × g for 10 minutes.
Pieces of harvested adipose tissues were washed in PBS, 10% formalin (Sigma-Aldrich), and held for at least 24 hours at 4℃, before being embedded in paraffin. Sections (6 to 8 µm) were cut on a rotary microtome (Leica RM2145, Leica Microsystems, Nussloch, Germany) fixed for 1 hour at 56℃, and then stored at RT. Before staining, sections were deparaffinized in xylenes. Tissue rehydration and all subsequent washes were performed by 25-minute incubations in a Zytomed wash buffer (Zytomed systems GmbH, Berlin, Germany). All incubations were completed at ambient temperature. For fluorescent immuno-staining, rehydrated tissue sections were pretreated with protein blocking in serum-free protein blocks (Dako, Glostrup, Denmark) and incubated with antibodies for 2 hours. Nuclear staining was attained through 10-minute incubation with Hoechst 33258 (Invitrogen, Carlsbad, CA, USA). Slides were mounted in Histomount (National Diagnostics, Atlanta, GA, USA), and observed under a fluorescence microscopy (BX61; Olympus, Tokyo, Japan) and a digital imaging system (DCF 500; Leica Microsystems).
Antibodies used in these studies were anti-CD140b (PDGFRβ, 1:50; BD Biosciences, San Jose, CA, USA), anti-CD146 (1:50; R&D Systems, Minneapolis, MN, USA), anti-CD90 (1:100; BD Biosciences), and anti-CD31 (1:100; BD Biosciences). All antibodies were diluted in an antibody diluent with background reducing components (Dako).
Cell surface antigen profiles of freshly isolated SVF cells were quantified by flow cytometry with a FACS.132223) Fat tissue was thoroughly minced with scissors and digested for 30 minutes in DMEM and 0.075% collagenase type I (Sigma Aldrich) on a rotator at 37℃. Mature adipocytes were eliminated by centrifugation (1,200 × g, ambient temperature, 10 minutes) and cell pellets were resuspended in blood cell lysis buffer, incubated for 10 minutes at RT and washed in PBS.
Freshly isolated cells from the SVF were maintained on ice, and stained for analytical flow cytometry and cell sorting experiments as previously described.24) Cell suspensions were centrifuged (1,200 × g, 10 minutes) and the cells were simultaneously stained with monoclonal mouse anti-human fluorochrome-conjugated antibodies: CD31-fluorescein isothiocyanate (FITC), CD34-FITC, CD90-FITC, CD140b-phycoerythrin (PE), CD146-PE, CD54-allophycocyanin (APC) (BD Biosciences), CD146-FITC, neuron-glial antigen 2 (NG2)-FITC, α-smooth muscle actin (αSMA)-PE, vascular endothelial growth factor receptor 2 (VEGFR2)-PE, C-X-C chemokine receptor type 4 (CXCR4)-PE, CD140b-APC (R&D systems), CD105-FITC, CD44-FITC (AbD Serotec, Kidlington, Oxford, UK), CD14-PE, CD45-PE (Dako), CD13-FITC (Novus Biological, Littleton, CO, USA), CD133-APC (Miltenyi Biotec, Auburn, CA, USA), and unconjugated antibodies (Desmin; Abcam, Cambridge, MA, USA). Analytical samples were fixed with 1% paraformaldehyde (PFA) for 10 minutes at RT. Four-color, 6-parameter data files were acquired on a 2-laser FACSCalibur flow cytometer (BD Biosciences) at a maximum of 10,000 events per second.
Cell sorting was performed using a 3-laser FACSAria II cell sorter (BD Biosciences).
Unfixed CD140b-PE stained samples were suspended in PBS, and 0.5% bovine serum albumin (BSA). Samples were continuously cooled to 4℃ and single cell sorting was performed at 70,000 events per second. Samples were collected into BD Falcon round-bottom polypropylene tubes (BD Biosciences) containing 1 mL of DMEM/Nutrient Mixture F-12 (DMEM/F12; Gibco) and plated in tissue culture dish (TPP; Techno Plastic Products, Trasadingen, Switzerland) at a density of 10,000 to 20,000 cell/cm2. Adherent sorted and unsorted cells were expanded in equal volumes of DMEM/F12 and supplemented with 10% FBS, and 100 U/mL penicillin (Sigma-Aldrich).
We evaluated the pericytic function of PDGFRβ+ cells that were collected from cultured ADSCs using FACS. We seeded human umbilical vein endothelial cells (HUVECs) and PDGFRβ+ ADSCs on the surface of a 100% Matrigel (BD Biosciences) in Endothelial Cell Growth Medium 2 (EGM2; Sigma-Aldrich) as previously reported.25) Briefly, Matrigel was added to a 24-well plate followed by incubation for 30 minutes at 37℃. PDGFRβ+ cells (passage 2) and HUVEC were labeled with anti-von Willebrand factor (vWF; Abcam) and anti-CD140b (PDGFRβ, BD Biosciences). They were then detached from the culture plate by trypsin and seeded onto the Matrigel in DMEM/F12 culture medium at a ratio of 1:1. In total, 1 × 105 cells were seeded in each well of 24-well plates. Capillary tube formation was investigated after five hours by fluorescence microscopy (IX51; Olympus), inverted microscopy (IX50; Olympus) and confocal laser scanning microscope (TCS SP8; Leica Microsystems) microscopy.
For osteogenesis, cells at 70% confluence were cultivated in DMEM, 10% FBS, 0.1 mM dexamethasone, 50 mg/mL L-ascorbic acid, and 10 mM b-glycerophosphate. After 14 days, cells were fixed in citrate fixative (citrate solution-acetone-37% formaldehyde) for 1 minute. For detection of alkaline phosphatase (ALP) activity, the fixed cells were incubated for 15 minutes in a mixture of Naphthol AS-BI alkaline solution (Sigma-Aldrich). Cells were then rinsed with deionized water and mounted in GVA aqueous mounting solution (Genemed, San Francisco, CA, USA).
For chondrogenesis, pellets were prepared by spinning down 3 × 105 cultured cells and grown in serum-free DMEM containing an insulin-transferrin selenious acid mix (Sigma-Aldrich), 50 mg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich), 100 mg/mL sodium pyruvate, 40 mg/mL L-proline (Sigma-Aldrich), 0.1 mM dexamethasone (Sigma-Aldrich), and 10 ng/mL transforming growth factor beta 1 (TGF-β1; R&D Systems). After 21 days, pellets were fixed in 10% formalin, dehydrated in ethanol, and embedded in paraffin. For the Safranin O staining, specimen sections (thickness, 6 µm) were deparaffinized with xylene and ethanol and Safranin O solution (Sigma-Aldrich) was applied for 5–10 minutes.
For adipogenic differentiation, cultured cells at 70% confluence were switched to DMEM, 10% FBS, 1 mM dexamethasone, 0.5 mM isobutylmethylxanthine, 60 mM indomethacin, and 170 mM insulin (Sigma-Aldrich). After 21 days, cells were fixed in 5% PFA at RT, washed in PBS, and incubated with oil red O for 10 minutes at RT for the detection of lipids.
All data are expressed as means ± standard deviations. Mann-Whitney U-test was used to compare branch points and tube numbers in Matrigel assay. IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis, and probability values of less than 0.05 were considered statistically significant.
White adipose tissue from the subcutaneous fat of the human lower leg is a richly vascularized tissue (Fig. 1A). Immunofluorescence staining using endothelial (CD31) and pericyte markers (PDGFRβ and CD146) showed the exclusively perivascular location of PDGFRβ+ cells in various sizes of the blood vessels (small artery, arteriole, and capillary) (Fig. 1B–D). Co-staining with a mesenchymal marker (CD90) showed that perivascular PDGFRβ+ cells also expressed mesenchymal markers in small arterioles (Fig. 1C and D), while CD90-positive cells and PDGFRβ+ cells were mutually exclusive in larger arteries (Fig. 1B).
Multilabel flow cytometric analysis revealed that PDGFRβ+ cells from fresh SVF of human adipose tissue expressed a higher level of a pericyte marker (αSMA), stem cell markers (CD34 and CXCR4), and mesenchymal markers (CD13, CD44, CD54, and CD90) than PDGFRβ– cells. PDGFRβ+ cells also expressed a slightly higher level of endothelial markers (CD31 and CD144) than PDGFRβ– cells (Fig. 2).24)
The CD45-positive cells are thought to include a mixture of leukocytes contained within the vasculature as well as cells resident in the adipose tissue.24) Staining of freshly isolated cells for PDGFRβ and other markers showed that there was a substantial number of perivascular mesenchymal stem-like (PDGFRβ+/CD90+/CD44+/CD45–) cell fractions in fresh SVF of human adipose tissue.
Plating of isolated cells from SVF on an uncoated tissue culture plastic resulted in selective adherence of cell population. Multilabel flow cytometric analysis of culture-expanded ADSCs revealed that FACS analysis from fresh SVF of human adipose tissue showed that a population of endothelial (CD31 and CD144) and leukocytic (CD45) marker-positive cells was greatly reduced (Fig. 3). Cultured PDGFRβ+ cells maintained the properties of PDGFRβ+ cells from fresh SVF except that they had a lower level of stem cell marker (CD34 and CXCR4)-positive cells. Cultured PDGFRβ+ cells expressed high levels of mesenchymal markers (CD13, CD44, CD54, and CD90), and a pericyte marker (αSMA). However, they expressed neither the endothelial markers (CD31 and CD144) nor the leukocytic marker (CD45).24)
When PDGFRβ+ ADSCs were cocultured with HUVEC, they demonstrated the formation of a tubular network (Fig. 4A). Tube number was higher (124.5 ± 13.2 per plate, p < 0.05) than that formed by HUVEC (97.5 ± 5.5) or ADSC (62.8 ± 5.6) only. At a higher magnification, they showed a pericytic location, where PDGFRβ+ ADSCs adhered to HUVEC (Fig. 4B). These results suggested that PDGFRβ+ ADSCs indeed possess a pericytic phenotype and stabilize the vascular tube-like network formed by HUVEC.
To examine whether these cells have a multilineage differentiation ability, PDGFRβ+ cells were induced to differentiate into the osteogenic, chondrogenic, and adipogenic lineages. During the analysis for osteogenic differentiation assayed by ALP staining, the PDGFRβ+ cells showed greater ALP staining (Fig. 5A). On the chondrogenic differentiation potential, verified by Safranin O staining, the PDGFRβ+ cells showed greater chondrogenic differentiation (Fig. 5B). After adipogenic induction for 3 days, the cell morphology changed from long spindle-shaped into a round or polygonal shape (data not shown). One week later, small bubble-shaped oil red O-staining lipid droplets appeared in some parts of the cells. The size of lipid droplets increased after 2 weeks, and most of the differentiated cells showed red lipid droplets throughout the cytoplasm (Fig. 5C). These results demonstrate that PDGFRβ+ cells have a multi-lineage differentiation ability.
In this study, we demonstrated that PDGFRβ+ cells are located in the perivascular area in fresh white adipose tissues of CLI patients and they coexpressed the MSC-like markers. We also showed that PDGFRβ+ cells from the stromal vascular fraction of human white adipose tissue had MSC-like characteristics and could differentiate into the multilineage mesenchymal tissues.
CLI, an end stage of lower limb ischemia due to PAD is considered as one of the main targets for cell therapy using stem cells and progenitor cells based on promising experiences with BM-MNCs,34567) PB-MNCs,89) and ADSCs.110)
Recently, the perivascular niche was discovered to be an important reservoir of MSCs in multiple developed organs (brain, spleen, liver, kidney, kidney glomeruli, lung, bone marrow, muscle, thymus, and pancreas), irrespective of their embryonic origin.14152627) Several studies have shown that cell populations obtained from the perivascular tissue exhibited the capacity of prolonged self-renewal and differentiated along mesenchymal cell lineages, making them defined as MSCs.24282930) Although perivascular progenitor cells are ubiquitous multipotent progenitors, isolation of perivascular progenitor cells for clinical application is not feasible from all human organs.18) Adipose tissue represents an attractive source of autologous adult stem cells for regenerative therapy owing to its abundance, surgical accessibility, and high content of multipotent MSCs.11) However, the development of an in vivo progenitor for culture-expanded MSC-like ADSCs remains elusive because of the complex organization of stromal cells surrounding the small vessels.13)
Coexpression of MSC-like markers in perivascular PDGFRβ+ cells suggests that perivascular niche could be an important reservoir of MSCs in white fat tissue, even in CLI patients. Traktuev et al.24) have shown that multipotent adipose-derived stromal cells (ASCs) also have a perivascular location and express pericyte markers. They showed that ASCs express the pericyte markers including CD140a, CD140b, NG2, and αSMA, and occasionally express CD146. The highly defined ASC population (CD34+/CD140a+/CD140b+/CD31–/CD45–/CD117–/CD144–) is the subset of adipose-derived cells, which possesses a majority of pericytic properties in the quiescent adipose tissue while harboring the ability to differentiate into multiple distinct lineages.
This study also demonstrates that PDGFRβ+ perivascular cells from ischemic adipose tissue have a good multilineage (osteogenic, chondrogenic and adipogenic) differentiation potential. However, the reason that neovascularization was inhibited in an ischemic condition in CLI patients with abundant PDGFRβ+ ADSC reservoirs is not clear. Further research needs to be performed to discover the signal that can awaken the PDGFRβ+ ADSC reservoir in CLI patients.
We carefully postulate that PDGFRβ+ cells from the SVF of adipose tissue can be used as a source of stem cells for autologous cell based treatment in CLI patients because (1) PDGFRβ+ cells in the SVF of adipose tissues are abundant and proliferative even in chronic CLI patients; (2) the pericytic localization of the PDGFRβ+ ADSCs and their direct contact with endothelial cells can be helpful to increase neovascularization in the ischemic limb; and (3) MSC-like characteristics of PDGFRβ+ ADSCs can be maintained during subsequent cell expansion in vitro. However, collecting SVF from other fat sites such as abdominal fat tissue might be better in clinical situation of CLI patients for saving ischemic limbs before major amputation.
In conclusion, we demonstrated that pericyte-like PDGFR-positive cells from the SVF of adipose tissue from CLI patients might have the potential to enhance vascularization. We also found that they could be amplified and cells characteristics could be preserved in in vitro culture. Considering the pericytic localization of PDGFRβ+ ADSCs and their direct contact with endothelial cells, PDGFRβ+ cells in the SVF of adipose tissue can be used as a source of stem cells for autologous cell-based treatment in CLI patients.
Figures and Tables
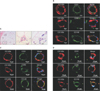 | Fig. 1Immunofluorescence of endothelial and perivascular markers in fresh white adipose tissue of human subjects. (A) H&E stain shows abundant vessels in the fat tissue including a small artery (large square), an arteriole (medium square), and a capillary (small square). The small artery (B), arteriole (C), and capillary (D) stained with indicated markers showed that platelet-derived growth factor receptor beta (PDGFRβ; CD140b)-positive (+) cells were located in the pericytic area while CD31 staining was exclusively visualized at the luminal surface for the small blood vessel. Costaining with a mesenchymal marker (CD90) showed that pericytic PDGFRβ+ cells also expressed the mesenchymal marker in small arterioles (C and D), while CD90-positive cells and PDGFRβ+ cells were mutually exclusive in the larger artery (B). |
 | Fig. 2Fluorescence-activated cell sorter analysis of fresh stromal vascular fraction. Platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells expressed a higher level of stem cell markers (CD34 and CXCR4) and mesenchymal markers (CD13, CD44, CD54, and CD90) than PDGFRβ– cells. Cell populations with a positive pericyte marker (α-smooth muscle actin [αSMA]) or endothelial markers (CD31 and CD144) were more abundant in PDGFRβ+ cells. VEGFR: vascular endothelial growth factor receptor, NG: neuron-glial antigen. |
 | Fig. 3Fluorescence-activated cell sorter analysis of culture-expanded adipose-derived stem cell from fresh stromal vascular fraction (SVF) of human adipose tissue. A population with endothelial (CD31 and CD144) and leukocytic (CD45) markers was substantially reduced. Cultured platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells maintained the feature of PDGFRβ+ cells from fresh SVF except that they had a lower level of stem cell marker (CD34 and CXCR4)-positive cells. Cultured PDGFRβ+ cells expressed high mesenchymal markers (CD13, CD44, CD54, and CD90) and a pericyte marker (α-smooth muscle actin [αSMA]). However, they did not express endothelial (CD31 and CD144) nor leukocytic (CD45) marker. VEGFR: vascular endothelial growth factor receptor, NG: neuron-glial antigen. |
 | Fig. 4Matrigel tube formation of fluorescence-activated cell sorter-sorted platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells. Human umbilical vein endothelial cells (HUVECs) and CD140b (+) cells were labeled with von Willebrand factor (vWF; green) and CD140b (red), respectively. Nuclei were labeled by DAPI stain (blue). (A) Tubular network formation was more abundant when PDGFRβ+ adipose-derived stem cells (ADSCs) were cocultured with HUVECs (c) than when HUVEC only (a) or ADSC only (b) were cultured. (B) When PDGFR beta-positive (PDGFRβ+) ADSCs were cocultured with HUVECs, they showed the pericytic location of PDGFRβ+ ADSCs (red) which adhered to HUVECs (green) when observed at higher magnification using a confocal microscope. |
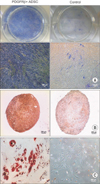 | Fig. 5Differentiation assay of fluorescence-activated cell sorter-sorted platelet-derived growth factor receptor beta-positive (PDGFRβ+) cells. (A) In osteogenic differentiation, which was assayed with alkaline phosphatase (ALP) staining, the PDGFRβ+ cells showed greater ALP staining. (B) In chondrogenic differentiation, which was verified by Safranin O staining, the PDGFRβ+ cells showed greater chondrogenic differentiation. (C) After adipogenic induction for 3 days, the cell morphology changed from long spindle-shape into a round or polygonal shape. One week later, small bubble-shaped oil red O-staining lipid droplets appeared in part of the cells. ADSC: adipose-derived stem cells. |
ACKNOWLEDGEMENTS
This study was supported by the Seoul National University Hospital Research Fund (No. 03-2012-0430) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. NRF-2012R1A1A2044067).
References
1. Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014; 16(2):245–257.

2. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD): TASC Working Group: TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000; 31(1 Pt 2):S1–S296.
3. Esato K, Hamano K, Li TS, et al. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant. 2002; 11(8):747–752.

4. Higashi Y, Kimura M, Hara K, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004; 109(10):1215–1218.

5. Saigawa T, Kato K, Ozawa T, et al. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004; 68(12):1189–1193.

6. Matoba S, Tatsumi T, Murohara T, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008; 156(5):1010–1018.

7. Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002; 360(9331):427–435.

8. Kinoshita M, Fujita Y, Katayama M, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012; 224(2):440–445.

9. Fujita Y, Kinoshita M, Furukawa Y, et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J. 2014; 78(2):490–501.

10. Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012; 76(7):1750–1760.

11. Gimble JM, Grayson W, Guilak F, Lopez MJ, Vunjak-Novakovic G. Adipose tissue as a stem cell source for musculoskeletal regeneration. Front Biosci (Schol Ed). 2011; 3:69–81.

12. Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005; 54(3):132–141.

13. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010; 77(1):22–30.

14. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008; 3(3):301–313.

15. Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008; 214(2):413–421.

17. Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005; 7(9):870–879.

18. Crisan M, Corselli M, Chen WC, Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012; 16(12):2851–2860.

19. Bhang SH, Cho SW, Lim JM, et al. Locally delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009; 27(8):1976–1986.

20. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005; 23(3):412–423.

21. Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004; 14(4-6):311–324.

22. Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008; 26(10):2682–2690.

23. Bagley RG, Rouleau C, Morgenbesser SD, et al. Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res. 2006; 71(3):163–174.

24. Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008; 102(1):77–85.

25. Ponce ML. Tube formation: an in vitro matrigel angiogenesis assay. Methods Mol Biol. 2009; 467:183–188.

26. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003; 18(4):696–704.

27. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119(Pt 11):2204–2213.

28. Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005; 96(9):930–938.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download