This article has been
cited by other articles in ScienceCentral.
Abstract
Background
A postoperative magnetic resonance imaging (MRI) is performed as a routine to assess decompression of the spinal cord as well as to evaluate postoperative complications. The purpose of this study is to analyze the efficacy of postoperative MRI for hematoma in spinal decompression surgery.
Methods
Between January 1, 2008 and January 31, 2015, 185 patients who underwent postoperative MRI after spinal decompression surgery were included in this study. We checked the history of the use of an anticoagulant or antiplatelet agent, withdrawal period, blood platelet count, and prothrombin time (international normalized ratio [INR]). We measured the total amount of suction drainage and duration until removal. We retrospectively reviewed the presence of hematoma and thecal sac compression. Postoperative prognosis was evaluated by a visual analog scale (VAS) and the Oswestry Disability Index (ODI).
Results
Hematomas were found on postoperative MRI scans in 97 out of 185 patients (52.4%). Thirty patients had a thecal sac compressing hematoma: 7 in the cervical spine, 1 in the thoracic spine, and 22 in the lumbar spine. The occurrence of hematoma did not show significant difference according to the use of an anticoagulant (p = 0.157). The blood platelet count, prothrombin time (INR), and suction drainage duration did not have a statistically significant correlation with the occurrence of hematoma (p = 0.562, p = 0.506, and p = 0.429, respectively). The total amount of suction drainage was significantly different according to the presence of hematoma (p = 0.022). The total 185 patients had a significant decrease in the postoperative VAS score (p < 0.001), and the diminution of VAS score was not significantly different according to the occurrence of hematoma (p = 0.243). Even in the cases of thecal sac compressing hematoma, the reduction of VAS score was not significantly different (p = 0.689).
Conclusions
Postoperative MRI for hematoma in spinal decompression surgery has little effect on prognosis or management. Therefore, indiscriminate postoperative MRI should be avoided and MRI should be performed depending on the patient's status.
Go to :

Keywords: Spinal epidural hematoma, Surgical decompression, Magnetic resonance imaging, Postoperative care
The majority of epidural hematoma, first described by Jackson
1) in 1869, is asymptomatic but care is needed because hematoma can lead paralysis.
2) Postoperative hematoma after spine surgery is mostly asymptomatic as well
2) and Sokolowski et al.
3) reported 58% of their patients developed asymptomatic hematoma. Extensive changes are frequently observed after spine surgery on magnetic resonance imaging (MRI), such as soft tissue swelling, fluid collection, hematoma formation, and residual compression of the thecal sac.
45) However, the clinical significance of these findings and the impact on the surgical decisionmaking process remain elusive.
6) Therefore, postoperative MRI for all patients is generally considered unnecessary.
7) However, postoperative MRI is performed as a routine to assess decompression of the spinal cord as well as to evaluate postoperative complications, and artefact reduction techniques can improve the image quality for early and improved detection of complications.
8) With increasing availability of MRI, postoperative MRI may be performed more readily than before, even without significant aggravation of symptoms or new deficits.
6) Moreover, MRI that does not affect the clinical course is sometimes performed for medicolegal reasons alone.
6) The purpose of this study is to analyze the efficacy of postoperative MRI for hematoma in spinal decompression surgery.
METHODS
Inclusion Criteria
Between January 2008 and January 2015, total spine surgeries were performed by a single surgeon in 749 patients. Of them, 185 patients who underwent postoperative MRI during hospitalization after spinal decompression surgery were reviewed retrospectively. They underwent MRI to check for cord decompression, complications, or unexpected symptoms. The postoperative MRI was generally performed just before discharge from the hospital; when a complication or unexpected symptom occurred, it was performed immediately. The inclusion criteria were as follows: (1) all elective spinal decompression surgeries were included regardless of the cause; (2) postoperative MRI should be performed before discharge; and (3) the level of spinal surgery included cervical, thoracic, and lumbar spines, regardless of the region. Emergent spinal operations were excluded because antiplatelet agent or anticoagulant therapy was not discontinued before surgery. Fusion-alone surgery was also excluded.
Measurement Methods by MRI
MRI was performed postoperatively using a 1.5 T MRI unit (Intera; Philips, Amsterdam, Netherlands) to obtain T2-weighted spin echo sagittal images, T1-weighted spin echo sagittal images, T1-weighted fat suppression spin echo sagittal images, T2-weighted spin echo axial images, T1-weighted spin echo axial images, T1-weighted gadolinium enhanced fat suppression spin echo sagittal images, and T1-weighted gadolinium enhanced fat suppression spin echo axial images. The slice thickness for sagittal and axial images was 4 mm.
Evaluated Variables
We calculated the duration from operation to postoperative MRI. We assessed the presence of hematoma and thecal sac compression and confirmed our findings with radiologists at the author's institution. The hematoma was defined as a space occupying epidural hematoma which is distinguishable in at least one plane from the axial or sagittal plane. Preoperative use of medication, such as anticoagulant or antiplatelet agent, was checked and the serum platelet count and prothrombin time (international normalized ratio [INR]) were assessed. We measured the total amount of suction drainage and duration until it was removed and checked the history of the use of sponge hemostatic agent. We checked the record of procedures, such as ultrasound-guided hematoma aspiration or hematoma evacuation surgery, in patients with hematoma. The visual analog scale (VAS) score and Oswestry Disability Index (ODI) were assessed preoperatively and 6 months postoperatively in the outpatient department.
Statistical Evaluation
The student t-test was used to evaluate differences between groups for continuous variables and the chi-square or Fisher exact test was used for the comparison of categorical variables. Turkey's test was used for post hoc analysis. The IBM SPSS ver. 23 (IBM Co., Armonk, NY, USA) was used for statistical analysis and p < 0.05 was considered to indicate statistical significance.
Go to :

RESULTS
Total 185 patients who underwent spinal decompression surgery were included in the study: 58 patients in the cervical spine (C-spine), 9 patients in the thoracic spine (T-spine), and 118 patients in the lumbar spine (L-spine) (
Table 1). Of those, 97 patients (52.4%) developed hematoma, which was confirmed on MRI (C-spine, T-spine, and L-spine: 27, 3, and 67, respectively). The occurrence of hematoma and operation site did not have statistically significant correlation (
p = 0.243). According to the operation type, the patients were categorized into 5 groups: decompression + anterior fusion; decompression + posterior fusion; laminectomy; laminoplasty; and discectomy. There was no significant difference in the occurrence of hematoma among the 5 groups (
p = 0.187). Thirty out of 97 patients (C-spine, T-spine, and L-spine: 7, 1, and 22, respectively) had a thecal sac compressing hematoma. It took an average of 10 days (standard deviation 13.92) from operation day to postoperative MRI, and this duration had no statistically significant correlation with the operation type (
p = 0.795).
Table 1
Demographic Data on Patients Who Underwent Spinal Decompression Surgery and Postoperative Magnetic Resonance Imaging
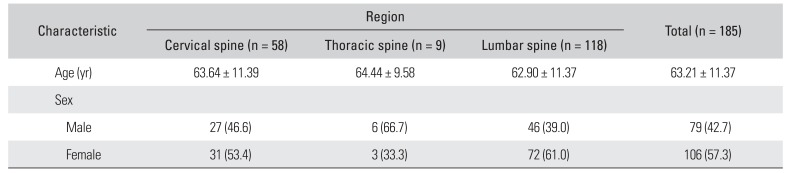
|
Characteristic |
Region |
Total (n = 185) |
|
Cervical spine (n = 58) |
Thoracic spine (n = 9) |
Lumbar spine (n = 118) |
|
Age (yr) |
63.64 ± 11.39 |
64.44 ± 9.58 |
62.90 ± 11.37 |
63.21 ± 11.37 |
|
Sex |
|
|
|
|
|
Male |
27 (46.6) |
6 (66.7) |
46 (39.0) |
79 (42.7) |
|
Female |
31 (53.4) |
3 (33.3) |
72 (61.0) |
106 (57.3) |

Ninety-eight out of 185 patients had a history of the use of an anticoagulant or antiplatelet agent and the average dosing period was 8.5 days before surgery. Hematoma was detected in 68 out of the 98 patients who took an anticoagulant or antiplatelet agent, and the incidence of hematoma in them was not significantly different (p = 0.157).
Postoperative VAS and ODI significantly decreased in all patients (
p < 0.001 for both) and the diminution of VAS and ODI was not significantly different depending on the presence of hematoma (
p = 0.243 and
p = 0.227, respectively) (
Table 2). Even in the case of thecal sac compressing hematoma, the diminution of VAS and ODI did not show significant difference (
p = 0.689 and
p = 0.068, respectively).
Table 2
Differences in VAS and ODI According to the Presence of Hematoma
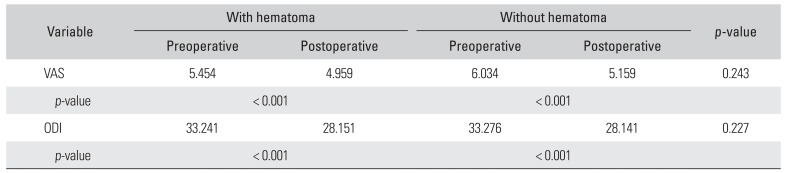
|
Variable |
With hematoma |
Without hematoma |
p-value |
|
Preoperative |
Postoperative |
Preoperative |
Postoperative |
|
VAS |
5.454 |
4.959 |
6.034 |
5.159 |
0.243 |
|
p-value |
< 0.001 |
< 0.001 |
|
|
ODI |
33.241 |
28.151 |
33.276 |
28.141 |
0.227 |
|
p-value |
< 0.001 |
< 0.001 |
|

Nine patients who had a thecal sac compressing hematoma complained of no change in numbness and prolonged pain for more than a week after operation. They underwent ultrasonography-guided hematoma aspiration and more than 1 mL (mean, 10 mL; standard deviation, 8.6) of hematoma was removed in all patients. The duration from operation to aspiration was an average of 8.3 days. Three patients (C-spine and L-spine: 1 and 2, respectively) underwent emergency hematoma evacuation surgery because they had neurologic symptoms, such as motor weakness (
Fig. 1).
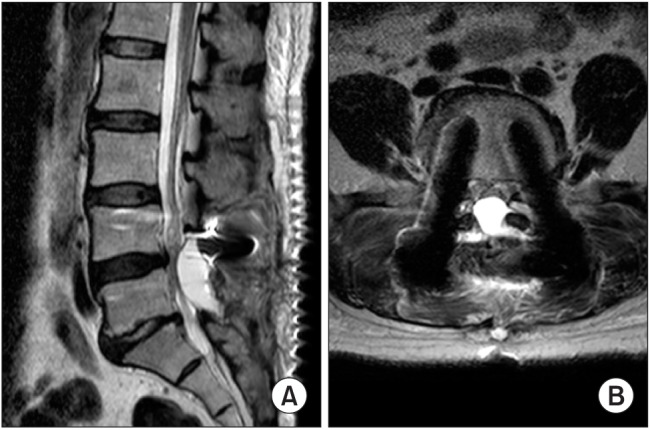 | Fig. 1(A) T2-weighted sagittal magnetic resonance imaging (MRI). Note the epidural hematoma at L5 level. (B) T2-weighted axial MRI. Note the pedicle screws and the epidural hematoma compressing the thecal sac and the nerve root.
|
All patients were classified into 3 groups: group 1, without hematoma group; group 2, with hematoma, without procedure group; and group3, with hematoma, with procedure group. The decrease in VAS and ODI showed no difference among 3 groups (
p = 0.371 and
p = 0.482, respectively). The preoperative serum platelet count and prothrombin time (INR) showed no difference among 3 groups in ANOVA (
p = 0.562 and
p = 0.506, respectively). The duration of suction drainage had no significant difference (
p = 0.429) among 3 groups, whereas the total amount of suction drainage showed a significant difference (
p = 0.022). On the post hoc analysis, there was a significant difference between group 1 and group 3 in Turkey's post hoc test (
p = 0.016) (
Table 3).
Table 3
Turkey's Post Hoc Test on the Coagulation Ability and Suction Drainage among 3 Groups
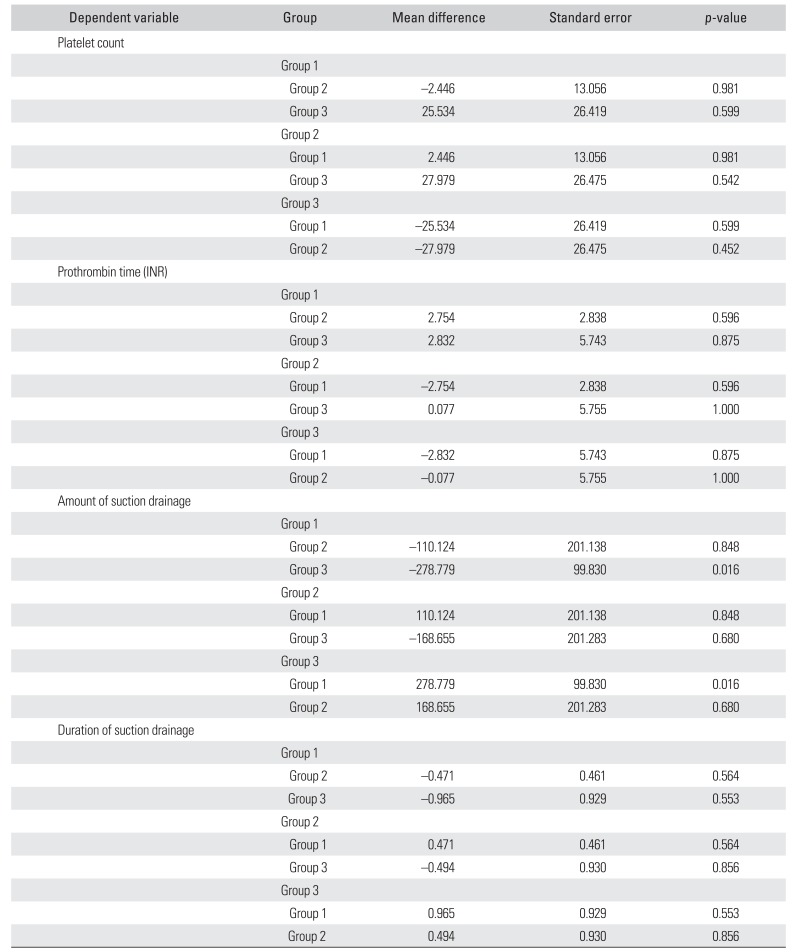
|
Dependent variable |
Group |
Mean difference |
Standard error |
p-value |
|
Platelet count |
|
|
|
|
|
Group 1 |
|
|
|
|
Group 2 |
−2.446 |
13.056 |
0.981 |
|
Group 3 |
25.534 |
26.419 |
0.599 |
|
Group 2 |
|
|
|
|
Group 1 |
2.446 |
13.056 |
0.981 |
|
Group 3 |
27.979 |
26.475 |
0.542 |
|
Group 3 |
|
|
|
|
Group 1 |
−25.534 |
26.419 |
0.599 |
|
Group 2 |
−27.979 |
26.475 |
0.452 |
|
Prothrombin time (INR) |
|
|
|
|
|
Group 1 |
|
|
|
|
Group 2 |
2.754 |
2.838 |
0.596 |
|
Group 3 |
2.832 |
5.743 |
0.875 |
|
Group 2 |
|
|
|
|
Group 1 |
−2.754 |
2.838 |
0.596 |
|
Group 3 |
0.077 |
5.755 |
1.000 |
|
Group 3 |
|
|
|
|
Group 1 |
−2.832 |
5.743 |
0.875 |
|
Group 2 |
−0.077 |
5.755 |
1.000 |
|
Amount of suction drainage |
|
|
|
|
|
Group 1 |
|
|
|
|
Group 2 |
−110.124 |
201.138 |
0.848 |
|
Group 3 |
−278.779 |
99.830 |
0.016 |
|
Group 2 |
|
|
|
|
Group 1 |
110.124 |
201.138 |
0.848 |
|
Group 3 |
−168.655 |
201.283 |
0.680 |
|
Group 3 |
|
|
|
|
Group 1 |
278.779 |
99.830 |
0.016 |
|
Group 2 |
168.655 |
201.283 |
0.680 |
|
Duration of suction drainage |
|
|
|
|
|
Group 1 |
|
|
|
|
Group 2 |
−0.471 |
0.461 |
0.564 |
|
Group 3 |
−0.965 |
0.929 |
0.553 |
|
Group 2 |
|
|
|
|
Group 1 |
0.471 |
0.461 |
0.564 |
|
Group 3 |
−0.494 |
0.930 |
0.856 |
|
Group 3 |
|
|
|
|
Group 1 |
0.965 |
0.929 |
0.553 |
|
Group 2 |
0.494 |
0.930 |
0.856 |

Gelfoam hemostatic agent (Floseal hemostatic matrix, Baxter International Inc., Deerfield, IL, USA) was used in 121 out of 185 patients, and hematoma occurred in 63 patients. There was no significant difference according to the use of Gelfoam (p = 0.0845).
Go to :

DISCUSSION
Postoperative imaging after spine surgery is certainly warranted if patients report new, aggravated or unexpected symptoms.
6) However, any decision on reoperation depends not on postoperative images but on symptoms.
6) In this study, the duration to postoperative MRI had no relation to hematoma, indicating that postoperative MRI was not performed for clinical purposes but routinely and indiscriminately. There are numerous reports that investigated the association between hematoma and anticoagulation therapy: the use of an anticoagulant and antiplatelet agent has not been associated with the formation of hematoma.
9101112131415) In this study, all operations were elective and all patients stopped taking anticoagulants before operation. The incidence of hematoma was not significantly different according to the use of an anticoagulant; however, interpretation of this result needs caution because all patients stopped medication for enough time before surgery. Kao et al.
16) reported that Gelfoam can cause mass effect with compression by absorbing blood and becoming surrounded with clot. In the current study, however, there was no correlation with Gelfoam and hematoma. The type and amount of Gelfoam may be related to hematoma. In addition, bleeding tendency is related not only with the anticoagulant therapy but also with the underlying disease of the patient. Future studies may shed additional light on this.
Postoperative VAS and ODI significantly decreased in all patients and the diminution of VAS and ODI was not significantly different according to the presence of hematoma. Most of the hematomas were asymptomatic. Three patients who had neurologic deficit underwent evacuation surgery and achieved improvement. In these cases, the decision on emergent operation depended not on postoperative imaging but on the symptom of the patients. Sokolowski et al.
3) reported that MRI evidence of hematoma was present in 58% of patients after lumbar decompression surgery and they were asymptomatic. Aono et al.
17) reported the incidence of hematoma evacuation after spine surgery ranged from 0.1% to 0.2%. Thus, postoperative MRI for hematoma has little effect on prognosis or management. A recent study revealed that postoperative hematoma would not be prevented by suction drainage regardless of drain diameter.
18)
However, the significant difference in the amount of drainage between the without hematoma group (group 1) and the with hematoma, with procedure group (group 3) indicates that postoperative MRI should be performed when the amount of drainage is greater than expected. Postoperative MRI has a critical diagnostic role in the evaluation of intracanalicular pedicle screw placement, incidental durotomy, and infection if patients report new neurologic deficit.
8) Neurologic recovery depends on the degree of deficit and the time of decompression; thus, early diagnosis and decompression surgery are critical.
19)
This study has several limitations. First, this study was retrospective in design and included only patients who underwent postoperative MRI, causing selection bias. Second, the size of hematoma and stage of thecal sac compression were not analyzed because MRI and picture archiving and communication system (PACS) of our institution cannot evaluate 3-dimentional volume. Third, factors that are related to the formation of hematoma were not addressed, such as the extent of surgical level, the time of operation, the duration from operation to postoperative MRI. Fourth, measurement and interpretation procedures were performed by multiple investigators raising the possibility of interobserver variation. Finally, this study focused on postoperative hematoma only, and thus lacks evidence of the efficacy of general spinal surgery, which we believe should be explored in future studies.
In conclusions, postoperative MRI for hematoma in spinal decompression surgery has little effect on prognosis or management. Therefore, caution should be exercise against indiscriminate use of MRI following spinal decompression surgery. We suggest that postoperative MRI be performed depending on the patient's status.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download