This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Piriformis syndrome (PS) is an uncommon disease characterized by symptoms resulting from compression/irritation of the sciatic nerve by the piriformis muscle. Uncertainty and controversy remain regarding the proper diagnosis and most effective form of treatment for PS. This study analyzes the diagnostic methods and efficacy of conservative and surgical treatments for PS.
Methods
From March 2006 to February 2013, we retrospectively reviewed 239 patients who were diagnosed with PS and screened them for eligibility according to our inclusion/exclusion criteria. All patients underwent various conservative treatments initially including activity modification, medications, physical therapy, local steroid injections into the piriformis muscle, and extracorporeal shock wave therapy for at least 3 months. We resected the piriformis muscle with/without neurolysis of the sciatic nerve in 12 patients who had intractable sciatica despite conservative treatment at least for 3 months. The average age of the patients (4 males and 8 females) was 61 years (range, 45 to 71 years). The average duration of symptoms before surgery was 22.1 months (range, 4 to 72 months), and the mean follow-up period was 22.7 months (range, 12 to 43 months). We evaluated the degree of pain and recorded the responses using a visual analog scale (VAS) preoperatively and 3 days and 12 months postoperatively.
Results
Buttock pain was more improved than sciatica with various conservative treatments. Compared with preoperatively, the VAS score was significantly decreased after the operation. Overall, satisfactory results were obtained in 10 patients (83%) after surgery.
Conclusions
PS is thought to be an exclusively clinical diagnosis, and if the diagnosis is performed correctly, surgery can be a good treatment option in patients with refractory sciatica despite appropriate conservative treatments.
Go to :

Keywords: Piriformis muscle, Sciatic nerve, Piriformis muscle syndrome
Piriformis syndrome (PS) is an elusive disease characterized by symptoms caused by compression/irritation of the sciatic nerve by the piriformis muscle as it exits the sciatic notch. PS commonly evokes the symptom of sciatica and is usually diagnosed only after excluding all other conditions originating from the back, buttocks or legs. Thus, it is likely that PS is often overlooked and probably represents the most common cause of extraspinal sciatica.
12)
Despite the abundance of related literature, the pathophysiology and diagnostic criteria of PS remain obscure. Brown et al.
3) emphasized contributory factors associated with PS including risky sports (e.g., long-distance running, cycling, and horse riding) and professions involving a prolonged seating position (e.g., truck drivers and taxi drivers). The diagnosis of PS is based largely on clinical symptoms, including buttock pain, pain aggravated by sitting, external tenderness near the greater sciatic notch, or physical tests—although they are not specific—when other causes of lumbar discogenic sciatica are excluded.
4) Although PS was once thought to be an exclusively clinical diagnosis, several reports have demonstrated the diagnostic value of electromyography (EMG), computed tomography (CT), magnetic resonance neurography (MRN), and local injections for PS.
156) However, no strict diagnostic criteria for PS exist because some patients with a normal CT, magnetic resonance imaging (MRI), myelography, or EMG complain of significant pain related to sciatica.
5)
Conservative treatment for PS includes activity modification (education on changing habitual postures or physical activities), the use of anti-inflammatory drugs, physical therapy, an injection of local anesthetics or corticosteroids, and botulinum neurotoxin injections.
78) The piriformis muscle is a short external rotator muscle of the hip joint that is stretched with internal rotation of the leg. Therefore, postures that evoke irritation of the sciatic nerve, such as squatting, leg twisting, pedal operating movement associated with machine sewing, and climbing, are avoided to relieve symptoms. Kirschner et al.
9) emphasized piriformis muscle stretching to correct the underlying pathology by relaxing a tight piriformis to relieve nerve compression. If conservative treatment is ineffective for symptom relief, surgical release of the piriformis and decompression of the sciatic nerve or neurolysis should be considered. Filler et al.
1) performed surgical resection of the piriformis muscle in 64 patients and obtained 82% initial and 76% long-term good or excellent outcomes. Nevertheless, diagnostic tools for PS remain obscure, and careful selection of patients for surgery is necessary to obtain good outcomes. Therefore, we aimed to retrospectively evaluate diagnostic methods for PS and the efficacy of surgery in our selected series.
METHODS
After approval of the Catholic University College of Medicine/St. Paul's Hospital Institutional Review Board (No. PC12RISI0044), we conducted a retrospective review of all patients with PS treated in our hospital from March 2006 to February 2013. Surgery was performed on 12 patients from among 239 patients who were diagnosed with PS using our inclusion/exclusion criteria (
Table 1). Of these, 43 patients had been transferred from the Department of Neurosurgery. Thirteen patients were diagnosed with failed back surgery syndrome.
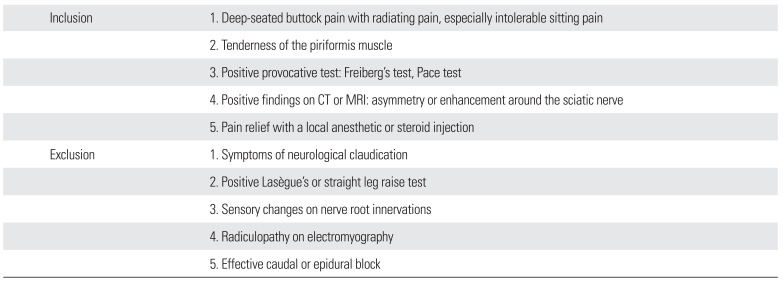
Table 1
Our Proposed Inclusion/Exclusion Criteria for Piriformis Syndrome
|
Inclusion |
1. Deep-seated buttock pain with radiating pain, especially intolerable sitting pain |
|
2. Tenderness of the piriformis muscle |
|
3. Positive provocative test: Freiberg's test, Pace test |
|
4. Positive findings on CT or MRI: asymmetry or enhancement around the sciatic nerve |
|
5. Pain relief with a local anesthetic or steroid injection |
|
Exclusion |
1. Symptoms of neurological claudication |
|
2. Positive Lasègue's or straight leg raise test |
|
3. Sensory changes on nerve root innervations |
|
4. Radiculopathy on electromyography |
|
5. Effective caudal or epidural block |

Surgery was indicated when pain (buttock pain or mainly sciatica) was not relieved by conservative measures, including education for habitual position or physical activity change, medication, physical therapy, local steroid injections on the piriformis muscle, or extracorporeal shock wave therapy (ESWT) for at least 3 months.
Twelve patients underwent surgery for PS and the average age of the patients (4 males and 8 females) who underwent surgery was 61 years (range, 45 to 71 years). The average duration of the symptoms before surgery was 22.1 months (range, 4 to 72 months), and the mean follow-up period was 22.7 months (range, 12 to 43 months). Of the 12 patients who had piriformis muscle resection with/without neurolysis, 8 had underlying pathologies including spinal stenosis; 5 had been managed by spinal block and 3 had undergone lumbar spinal surgery, but their symptoms had not been relieved. Three patients had a long-time, occupation-related, habitual sitting position, and 1 patient had a history of sacral fracture. One patient had noninfectious sacroiliitis, which was deemed the cause of disease. We evaluated buttock pain with/without sciatica using a visual analog scale (VAS; 0, no pain; 10, maximum pain) and recorded the responses preoperatively, and at 3 days and 12 months postoperatively. Statistical analysis was carried out using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Kruskal-Wallis and Mann-Whitney U-tests were used for post hoc analysis to compare changes in VAS for pain over time.
Surgical Approach
Surgery was performed in the lateral decubitus position. A curvilinear skin incision was made > 10 cm over the greater trochanter. The gluteus maximus was divided in the direction of its fibers by blunt dissection, and the fascia lata was incised in continuity where it overlaid the trochanter; we then removed the trochanteric bursa. The piriformis muscle was inserted into the posterior aspect of the greater trochanter as tendinous nature and was located above the obturator internus tendon (
Fig. 1A). The sciatic nerve was explored and found to pass anteriorly to the piriformis muscle in all cases. Additionally, we divided the tight piriformis tendon at the insertion site at its tendinous portion. The proximal portion of the muscle was retracted when the leg was internally rotated after its division. Neurolysis around the sciatic nerve into the sciatic notch was performed in two cases of a severely adherent sciatic nerve. Severely dilated and engorged epineurial vessels were found in two cases with intractable sciatica (
Fig. 1B). Hemovac drain insertion was used routinely. After surgery, patient activity with the assistance of a cane was encouraged to relieve pain from the gluteal muscle repair.
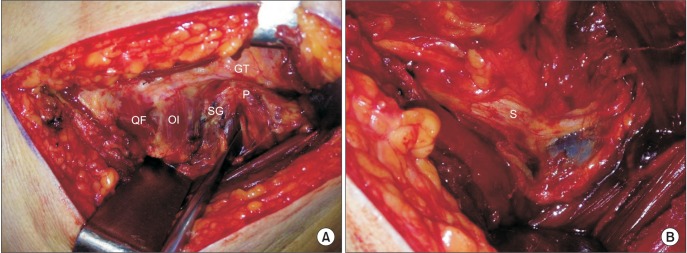 | Fig. 1Intraoperative photographs. (A) The tensor fascia lata was incised in continuity where it overlays the trochanter, and the piriformis muscle (P) was inserted into the posterior aspect of the greater trochanter (GT) and located above the superior gemellus muscle (SG) and obturator internus tendon (OI). (B) After division of the tight piriformis tendon at the insertion site at its tendinous portion, a distally adherent sciatic nerve (S) with engorged epineurial vessels was observed. QF: quadratus femoris.
|
Clinical Evaluation
The diagnostic procedure involved a detailed physical examination, including a palpation test for tenderness over the origin (sacroiliac joint) or insertion of the short external rotators behind the trochanter (
Fig. 2). The clinical examination also included several provocation tests for pain and weakness on resisted abduction and external rotation of the thigh in a sitting position (Pace test), pain on forced passive internal rotation of the extended thigh (Freiberg's test), and buttock and leg pain during passive straight leg raising (Lasègue's sign). In addition to electromyography, various imaging studies—including CT, MRI, and ultrasonography— were performed, and a steroid injection into the piriformis muscle was carried out for diagnostic treatment. To exclude sciatica caused by spinal problems, repeated caudal or epidural block was performed. Patients who had greater than 50% relief of symptoms of sciatica after caudal block were excluded from having a diagnosis of PS. Asymmetry of the piriformis muscle on CT or MRI was considered a positive finding of PS. An electromyographic finding of radiculopathy was attributed to spinal root compression, not to PS (
Fig. 3).
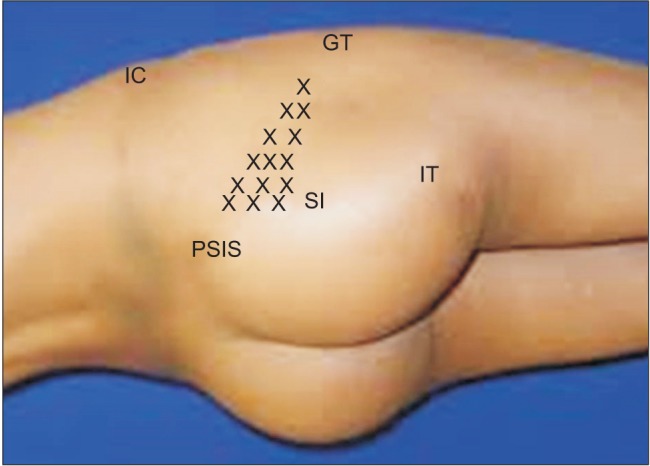 | Fig. 2Physical examination. The location of a tender point (x) in the gluteal area, particularly on the iliac side of the sacroiliac joint (SI), is consistent with that of the piriformis muscle. IC: iliac crest, IT: ischial tuberosity, GT: greater trochanter, PSIS: posterior superior iliac spine.
|
 | Fig. 3Algorithm for the diagnosis and treatment of piriformis syndrome (PS). CT: computed tomography, MRI: magnetic resonance imaging, EMG: electromyography, PT: physical therapy, ESWT: extracorporeal shock wave therapy.
|
Go to :

RESULTS
For the diagnosis of PS, physical examinations, including several provocative tests, radiographic studies, such as plain X-ray, CT or MRI, EMG, or an injection were performed. When a diagnosis of PS was suspected, various conservative treatments were initially performed in all patients and generally provided good results (
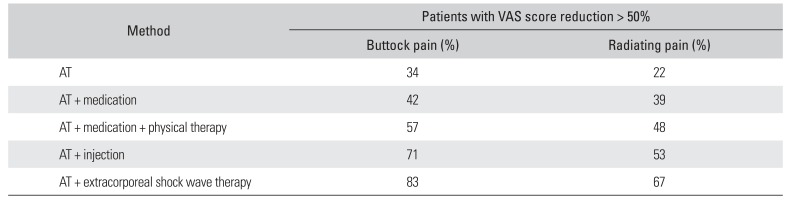
Table 2). Buttock pain was more efficiently relieved than sciatica by the conservative treatments. ESWT was the most effective method for reducing buttock pain. Of the 239 patients, 12 patients who were refractory to conservative treatment underwent surgical treatment.
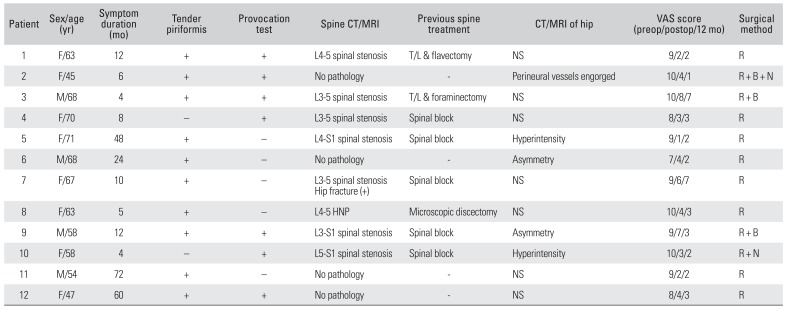
Table 3 summarizes the clinical features and the results of surgical treatment. On physical examination, tenderness in the gluteal area, particularly on the iliac side of the sacroiliac joint, was detected in 10 patients (83%). Pain provocative tests—such as the Freiberg's and Pace tests—were positive in 7 patients (58%). Electrodiagnostic testing showed no specific findings (delayed H-reflex at the flexion adduction internal rotation position) suggesting PS as opposed to other pathologies, such as lumbosacral radiculopathy, sciatic nerve palsy, or posterior cutaneous neuropathy of the thigh. Asymmetry of the piriformis muscle or hyperintensity around the sciatic nerve on CT and MRI was detected in only 5 patients (42%) (
Fig. 4). Three patients had occupations that involved sitting for a long duration, such as sewing or driving.
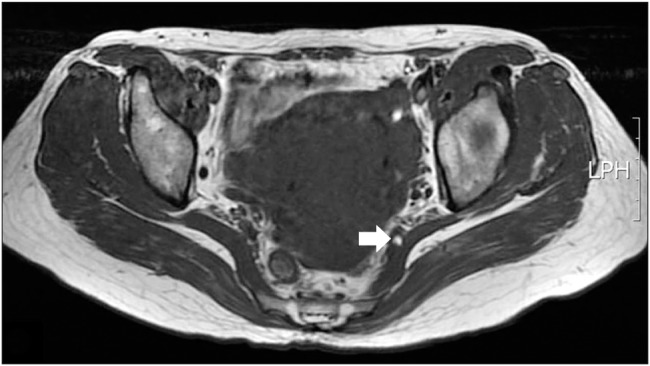 | Fig. 4Magnetic resonance imaging shows that the left sciatic nerve is entrapped, and perineural vessels are engorged (arrow) by the piriformis muscle in a 45-year-old female. LPH: lumbopelvic hip.
|
Table 2
Results of Conservative Treatments
|
Method |
Patients with VAS score reduction > 50% |
|
Buttock pain (%) |
Radiating pain (%) |
|
AT |
34 |
22 |
|
AT + medication |
42 |
39 |
|
AT + medication + physical therapy |
57 |
48 |
|
AT + injection |
71 |
53 |
|
AT + extracorporeal shock wave therapy |
83 |
67 |

Table 3
Results of Diagnostic Investigation and Surgical Treatment
|
Patient |
Sex/age (yr) |
Symptom duration (mo) |
Tender piriformis |
Provocation test |
Spine CT/MRI |
Previous spine treatment |
CT/MRI of hip |
VAS score (preop/postop/12 mo) |
Surgical method |
|
1 |
F/63 |
12 |
+ |
+ |
L4-5 spinal stenosis |
T/L & flavectomy |
NS |
9/2/2 |
R |
|
2 |
F/45 |
6 |
+ |
+ |
No pathology |
- |
Perineural vessels engorged |
10/4/1 |
R + B + N |
|
3 |
M/68 |
4 |
+ |
+ |
L3-5 spinal stenosis |
T/L & foraminectomy |
NS |
10/8/7 |
R + B |
|
4 |
F/70 |
8 |
− |
+ |
L3-5 spinal stenosis |
Spinal block |
NS |
8/3/3 |
R |
|
5 |
F/71 |
48 |
+ |
− |
L4-S1 spinal stenosis |
Spinal block |
Hyperintensity |
9/1/2 |
R |
|
6 |
M/68 |
24 |
+ |
− |
No pathology |
- |
Asymmetry |
7/4/2 |
R |
|
7 |
F/67 |
10 |
+ |
− |
L3-5 spinal stenosis |
Spinal block |
NS |
9/6/7 |
R |
|
8 |
F/63 |
5 |
+ |
− |
L4-5 HNP |
Microscopic discectomy |
NS |
10/4/3 |
R |
|
9 |
M/58 |
12 |
+ |
+ |
L3-S1 spinal stenosis |
Spinal block |
Asymmetry |
9/7/3 |
R + B |
|
10 |
F/58 |
4 |
− |
+ |
L5-S1 spinal stenosis |
Spinal block |
Hyperintensity |
10/3/2 |
R + N |
|
11 |
M/54 |
72 |
+ |
− |
No pathology |
- |
NS |
9/2/2 |
R |
|
12 |
F/47 |
60 |
+ |
+ |
No pathology |
- |
NS |
8/4/3 |
R |

Of the 12 patients undergoing surgical resection of the piriformis muscle, neurolysis was performed in 2 patients due to a severely adherent or scarred sciatic nerve. Engorged epineurial vessels around the sciatic nerve shrank spontaneously after resection of the piriformis muscle. A hypertrophic trochanteric bursa was excised in 3 patients. The average length of the skin incision was 9.5 cm, and the average amount of postoperative bleeding was 24.5 mL. There were no postoperative complications including hematoma, infection, delayed wound healing, scar formation, and myositis ossificans. The average duration of hospitalization was 5.3 days (range, 3 to 9 days).
Compared with preoperatively (mean VAS score, 9), the VAS scores significantly decreased both immediately after surgery (mean VAS score, 4) and at the 12-month follow-up (mean VAS score, 3.1) (
Table 4). Sciatica was almost resolved in 9 patients within 3 postoperative days. Persistent buttock pain after surgery was present in 3 patients. Among them, 1 patient had symptom relief after 12 months. Overall, satisfactory results were obtained in 10 patients (83%).
Table 4
Visual Analog Scale (VAS) at Preoperative, Immediate Postoperative, and 12 Months Follow-up

|
Variable |
Preoperative |
Postoperative |
At 12 mo |
|
VAS for pain (mean ± standard deviation) |
9.00 ± 0.91 |
4.00 ± 2.00 |
3.10 ± 1.85 |
|
p-value (vs. preoperative) |
- |
< 0.001 |
< 0.001 |

Go to :

DISCUSSION
The diagnosis of PS is mostly elusive and remains controversial due to the lack of consistent objective diagnostic criteria. The differential diagnoses should include herniation of the nucleus pulposus (HNP), myofascial pain, sacroiliitis, trochanteric bursitis, or any other sciatic nerve-impinging conditions. In this study, the diagnosis of PS could be established with the patient's history, a careful physical examination, and a local injection of the piriformis muscle when no other etiological findings were identified on EMG or imaging studies, including CT and MRI (
Fig. 3). The piriformis muscle is innervated from the L5 to S2 roots. Because lumbosacral HNP or spinal stenosis commonly occurs at the L4-5 or L5-S1 intervertebral space, it is important to determine whether the pain originates from the root or peripheral nerve.
10) In patients with PS, symptoms of neurological claudication are rare, while pain aggravation by a position change or prolonged sitting is frequently observed in them. Specific sensory changes in dermatomes or muscle weakness can help to exclude PS. Based on our cases, we suggest that the three cardinal symptoms of PS are buttock pain, radiating pain to the posterior thigh above the knee, and pain aggravated by position changes or prolonged sitting. The piriformis muscle can be firm and hard to palpation from the greater sciatic notch to the posterior aspect of the greater trochanter. In physical examination, tenderness of the piriformis muscle (83%) is the most consistent finding.
Although EMG is often normal in patients with PS, continuous compression may result in abnormal spontaneous activity of the muscles innervated by the sciatic nerve, including a delay in the H-reflex with the affected leg in a flexed, adducted, and internally rotated position.
11) In our study, electrodiagnostic testing was not helpful for the diagnosis of PS but useful in ruling out other causes with similar symptoms, such as lumbosacral radiculopathy.
Diagnostic imaging modalities, including CT, MRI, and MRN, have been used in many studies to diagnose PS.
12131415) However, these studies are limited to cases showing atypical anatomy, including asymmetry of the piriformis muscle or hyperintensity of the sciatic nerve; these conditions accounted for only 5 of the 12 surgical patients (42%) in our study. Sayson et al.
16) and Barton
17) found that preoperative MRI failed to identify atypical anatomy that was found intraoperatively. MRN is a relatively new technique that was developed specifically to enhance the imaging of nerves. Filler et al.
1) used MRN to prospectively investigate 87 patients with sciatica-like pain in whom either standard testing had failed to yield a diagnosis or who had failed lumbar disc surgery; 67% of this group was diagnosed with PS. However, Tiel
18) pointed out methodological and technical problems of MRN.
Some reports have suggested diagnostic criteria for PS.
1920) Recently, Michel et al.
21) proposed to use a clinical scoring system: PS can be considered “probable” with a score of 8 or more out of 12 points. The scoring system helps to exclude spine problems originating from sciatica. However, it includes negative findings for spinal disease, such as no lower back pain, painless axial spinal palpation, negative Lasègue's maneuver, or absence of perineal irritation (4 points). A scoring system including negative findings for spinal problems can result in overdiagnosis of PS. In addition, the scoring system involves obscure physical tests that provoke buttock pain or sciatica by stretching or resisted contraction, and it does not include some diagnostic tests, such as local steroid injection, which is a widely used tool for establishing the diagnosis.
We propose a new set of diagnostic criteria for PS (
Table 1). They are comprised of 5 items: buttock pain with/without sciatica during prolonged sitting; tenderness of the piriformis muscle; positive provocative tests; positive findings on CT or MRI; and pain relief with a local injection. PS is diagnosed when at least 4 criteria are met. Tenderness of the piriformis muscle was the most consistent finding in our series. Additionally, caudal or epidural block was tried at least once before surgery in our study.
We suggest to exclude a diagnosis of PS with any of the following findings: symptoms of neurologic claudication; positive Lasègue's or straight leg raise test; sensory changes on nerve root innervation; radiculopathy on EMG; or an effective caudal or epidural block. The sciatic nerve compression by the piriformis muscle or surrounding fibrous bands is different from the nerve root compression of spinal origin. Absent clinical findings due to nerve root compression is important in the diagnosis of PS.
Several methods for the treatment of PS exist. However, the results are variable, and no particular treatment has been recommended. Initial nonoperative treatment of PS includes medications (nonsteroidal anti-inflammatory drugs, muscle relaxants, and other medications effective in neuropathic pain, such as pregabalin or gabapentin), physiotherapy, ESWT, injections of local anesthetics and corticosteroids, and the more recently investigated option of botulinum neurotoxin injections.
8) In our study, ESWT was applied in patients with buttock pain more than twice with an interval of 1 week until pain subsides significantly. ESWT was undertaken with 2,000 pulses each time at 1 week interval totaling 4,000 to 6,000 pulses. Our clinical results demonstrated that the most effective modality in treatment of PS for reducing buttock pain was ESWT.
We had satisfactory clinical results after release of the piriformis muscle and neurolysis of sciatic nerve in patients with refractory sciatica that fail to respond successfully to conservative treatments. Intraoperatively, identification of the piriformis muscle among short external rotator muscles and posterior retraction after complete resection of the muscle are important. Careful dissection of fibrous tissues around the sciatic nerve is also essential to avoid damaging the nerve proper or the dilated vaso nervorum.
Deep gluteal syndrome (DGS) is a disease entity that is characterized by pain or dysesthesia in the buttock area, hip, or posterior thigh and/or radicular pain due to a nondiscogenic sciatic nerve entrapment in the subgluteal space.
22) Its main pathology is fibrous bands around the sciatic nerve formed by various pathological conditions, such as piriformis syndrome, obturator internus/gemellus syndrome, or ischiofemoral impingement. The causes of DGS include traumatic, iatrogenic, inflammatory/infectious, vascular, and gynecological processes, and tumors/pseudotumors. Therefore, the treatment of DGS is decompression of the sciatic nerve via open or endoscopic surgery. It is not clear whether resection of the piriformis muscle has additional benefits over decompression of the sciatic nerve in PS. However, the recurrence of sciatic nerve adhesions can be avoided when the muscle is resected.
1) Endoscopic surgery for adhesiolysis of the sciatic nerve has several advantages over open methods, including less invasiveness and less postoperative pain.
23) However, endoscopic sciatic nerve release is technically demanding and has limited efficacy for release of a severely adherent sciatic nerve. Considering that no prospective, randomized trial has evaluated surgical treatment outcomes of PS, the important finding of our study was the significant decrease in symptoms after surgery. To achieve good results, the indications for surgery (no response to physical therapy and at least one injection) should be determined upon proper diagnosis (based exclusively on clinical and spine evaluations). Surgery is an important treatment option for unresolved PS because of its low morbidity and simplicity.
To overcome the limitations of our work, a prospective study with a greater number of cases and a longer follow-up period should be performed to establish the gold standard methods for the diagnosis and treatment of PS; furthermore, several modalities for diagnosis of PS should be developed.
In conclusions, the diagnosis of PS is obscure and elusive, but a systematic approach is helpful. If a diagnosis is determined correctly, surgical treatment can be a good option in patients with refractory pain, particularly sciatica, despite application of appropriate conservative treatment modalities.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download