Abstract
Rotational acetabular osteotomy (RAO) is a well-established surgical procedure for patients with acetabular dysplasia, and excellent long-term results have been reported. However, RAO is technically demanding and precise execution of this procedure requires experience with this surgery. The usefulness of computer navigation in RAO includes its ability to perform three-dimensional (3D) preoperative planning, enable safe osteotomy even with a poor visual field, reduce exposure to radiation from intraoperative fluoroscopy, and display the tip position of the chisel in real time, which is educationally useful as it allows staff other than the operator to follow the progress of the surgery. In our results comparing 23 hips that underwent RAO with navigation and 23 hips operated on without navigation, no significant difference in radiological assessment was observed. However, no perioperative complications were observed in the navigation group whereas one case of transient femoral nerve palsy was observed in non-navigation group. A more accurate and safer RAO can be performed using 3D preoperative planning and intraoperative assistance with a computed tomography-based navigation system.
Rotational acetabular osteotomy (RAO) is a well-established surgical procedure that aims at joint preservation.1) Excellent long-term results of this procedure have been reported for early-stage hip osteoarthritis with acetabular dysplasia.23) However, RAO is technically demanding and precise execution requires experience in performing this surgery; even experienced surgeons sometimes encounter difficulties, especially in cases with severe acetabular dysplasia. Ideally, precise preoperative planning with consideration of the three-dimensional (3D) structure of the pelvis would be utilized. However, two-dimensional plain radiographs are frequently used for RAO preoperative planning.
Recently, 3D planning using computer software and full-scale 3D models based on information obtained from computed tomography (CT) data has been utilized. For total hip arthroplasty (THA), the technology for 3D preoperative planning and intraoperative computer assistance is considered to be well established. However, this technology is still in the developmental stage and has not been adopted for RAO as widely as the computer-assistance technology in use for THA. Since August 2011, we have performed 3D preoperative planning for RAO using computer software and used CT-based navigation in the implementation of preoperative planning.
In this article, we introduce RAO using CT-based navigation performed in our department and describe the short-term outcomes. The procedures described in this article were approved by the authors' Institutional Review Board. All patients and their families were informed that data from the case would be submitted for publication, and gave their consent.
CT scans of the pelvis and femur with 1.5 mm slice thickness were performed before each surgery. CT data were transferred to 3D template software (ZedHip; LEXI Co., Tokyo, Japan) in Digital Imaging and Communications in Medicine (DICOM) format to create a 3D bone model. The 3D-shape data created were converted to the Standard Template Library (STL) format and transferred to modeling software (FreeForm; Sensable, Wilmington, MA, USA), which was used to perform the preoperative planning. For intraoperative assistance, the preoperative plan was transferred to a CT-based navigation system (OrthoMap 3D Navigation System; Stryker Orthopaedics, Mahwah, NJ, USA) in STL format, and the osteotomy was performed using CT-based navigation according to the preoperative plan. For preoperative planning, the spherical osteotomy line of the acetabulum was planned so that the osteotomy line passed approximately 25 mm proximal to the upper acetabular margin and from the innominate groove of the ischium to the middle point between the greater sciatic notch and the posterior acetabular margin, with the center of the sphere near the center of the femoral head (or the center of the hip joint). The line was planned so that it ran distal of the anterior inferior iliac spine to the center of the pubis and slightly through the iliac inner cortex to avoid cutting into the joint. The diameter of the sphere was generally 80 to 90 mm, and differed among the cases. The amount of rotation by the acetabular bone fragment obtained by osteotomy was planned such that it would be able to rotate laterally until the acetabular roof obliquity angle4) became 0°. If the anterior part of the acetabulum was insufficiently covered, the planned amount of anterior rotation was adjusted so that the correct amount of coverage was obtained (Fig. 1).
Intraoperative Assistance (Supplement 1
RAO was performed with the patient in the lateral position, and the navigation device placed on the cranial side of the operating table. We used the transtrochanteric approach because this enabled sufficient hip exposure and facilitated the surgery with the assistance of computer navigation.
After the hip was exposed, two pins were inserted into the iliac crest, and a pelvic tracker attached. After confirming that the pelvic tracker was firmly fixed in place, the pelvic shape was registered by performing surface matching with 30 or more points following point pair matching. The registration was confirmed by touching the bone surface with a pointer. If the accuracy was insufficient, measures such as increasing the number of points for surface matching or repeating the registration were undertaken. Following registration, the tracker was installed on a high-speed drill, the tip of which was registered. The osteotomy line was marked with the high-speed drill according to the preoperative plan, while confirmation of the tip position of the high-speed drill was noted on a computer screen (Fig. 2). Following this, a curved chisel was registered and its tip position confirmed using the same procedure used with the high-speed drill, and osteotomy of the cancellous bone and the iliac inner cortex was performed according to the preoperative plan (Fig. 3). Since we did not dissect the tendon of the rectus femoris muscle, osteotomy of the pubis had to be performed in a poor visual field, which is technically demanding, but possible to perform safely by confirming the tip position of the chisel on the computer screen. To increase the safety of the operation, a warning alarm was set; the computer screen turned red if the chisel tip went further than the preoperatively planned osteotomy line. This allowed for any differences from the preoperative plan to be confirmed during surgery with navigation, and the osteotomy could be performed with appropriate modifications. Since the position of the dissected and rotated bone fragment could not be confirmed in real time, we confirmed if the position to which the bone fragment had moved was the same as that in the preoperative plan by temporarily fixing the bone fragment after it was rotated to the same level as in the preoperative plan, while successively touching the surface of the rotated bone fragment with the pointer (Fig. 4). The rotated bone fragment was fixed using three polylactic acid absorbable screws, 4.5 mm in diameter. The position of the rotated bone fragment was also confirmed with the pointer after fixation, without using intraoperative fluoroscopy (Fig. 5).
Twenty-three hips of 22 patients (20 females and 2 males) who underwent RAO using CT-based navigation after August 2011 (the navigation group) and 23 hips of 20 consecutive patients (19 females and 1 male) who underwent RAO without using CT-based navigation before August 2011 (non-navigation group) were investigated. For the radiological assessment, the center-edge angle, acetabular head index, acetabular roof obliquity angle, and acetabular angle were measured on preoperative and postoperative plain radiographs of the hip. The operative time, operative blood loss, and intraoperative fluoroscopy time were investigated in all patients, with the exception of those who also underwent femoral osteotomy (two hips in the navigation group and six hips in the non-navigation group). The presence or absence of perioperative complications in each group was also investigated. Statistical analysis of differences between the two groups was performed using a t-test, and the significance level was set at 5%.
Radiologically, a significant improvement in the center-edge angle, acetabular head index, acetabular roof obliquity angle, and acetabular angle was observed after surgery in both the navigation and non-navigation groups (p < 0.05). No significant difference was observed in these four radiographic measurements after surgery between the navigation and non-navigation groups (Table 1). Average operative time was 142 ± 34 minutes in the navigation group and 107 ± 43 minutes in the non-navigation group (p = 0.25), and the average operative blood loss was 589 ± 377 mL in the navigation group and 428 ± 281 mL in the non-navigation group (p = 0.11). The average intraoperative fluoroscopy time was 5 ± 10 seconds in the navigation group and 44 ± 21 seconds in the non-navigation group, with the former being significantly shorter (p < 0.001). No perioperative complications were observed in the navigation group, but transient femoral nerve palsy was observed in one patient in the non-navigation group. The femoral nerve palsy of this patient had recovered within one and a half years after the surgery under only observation.
With advances in technology, computer navigation systems have been developed as surgery support tools, and their usefulness has been reported. Such navigation systems are classified into three types: CT-based navigation, imageless navigation, and fluoroscopy-based navigation. The features of CT-based navigation include its ability to allow detailed preoperative planning using CT images taken before surgery. The disadvantages include exposure to radiation associated with preoperative CT imaging. The accuracy of CT-based navigation, based on the error in cup placement in THA, has been reported to be 1.8° ± 1.6° for the inclination angle of and the 1.2° ± 1.1° for the anteversion angle.5)
In recent years, there have been several reports on the use of computer navigation for periacetabular osteotomy. Akiyama et al.6) reported use of CT-based navigation for a curved periacetabular osteotomy in three patients and that it increased the accuracy and safety of the surgical procedure and decreased the incidence of intraoperative complications. Langlotz et al.7) reported 14 patients who underwent Bernese periacetabular osteotomy using CT-based navigation, and found that, although operative time and operative blood loss increased compared with the traditional approach, no intraoperative/postoperative complications were observed and an accurate and safe pelvic osteotomy could be performed. However, Hsieh et al.8) reported that in patients who underwent periacetabular osteotomy, no significant differences with regard to operative blood loss, blood transfusion requirement, functional improvement, or radiographic results were observed between the 18 patients in the CT-based navigation group and the 18 patients in the non-navigation group, and concluded that computer navigation offers little additional benefit when the surgery is performed by an experienced surgeon. However, they also presented the argument that when using CT-based navigation the radiation exposure from intraoperative fluoroscopy is decreased and that the surgery is facilitated by confirming the osteotomy site in real time. Tokunaga and Watanabe9) verified the accuracy of the surface-matching registration on the inner cortex of the pelvis in CT-based navigation (OrthoMap 3D Navigation System; Stryker Orthopaedics) and reported that the mean error was ~1 mm, which is clinically acceptable. They also concluded that the accuracy of the surgical procedure and the reduction of operative time and stress during curved periacetabular osteotomy could be improved using CT-based navigation.
Our results are also in agreement with many previous findings. No significant differences were observed in the radiological assessments between the navigation and non-navigation groups, but there were also no perioperative complications in the navigation group. Therefore, we consider osteotomy using navigation to be a safer option than traditional osteotomy. Additionally, no significant difference was observed with regard to the operative time or blood loss between the two groups, although there was a tendency toward a longer operative time and greater operative blood loss in the navigation group. We think that while the use of computer navigation facilitates osteotomy, the operative time tends to be longer because of the time required for tracker installation and registration, and operative blood loss tends to increase correspondingly. However, although the operative time was longer and the blood loss was greater in the first 10 patients in the navigation group, due to the learning curve for introducing the navigation technique, the operative time and blood loss have been reduced. The sample size necessary to account for the learning curve in the use of navigation should be examined in the future studies. The intraoperative fluoroscopy time was significantly shorter in the navigation group. Intraoperative fluoroscopy was used for confirmation in the first five patients in the navigation group, but was not used in subsequent patients. In this study, consecutive patients who underwent RAO before starting the use of navigation were set as a control (historical control), and since the follow-up duration differed significantly between the navigation and non-navigation groups, the clinical results were not investigated. Since the follow-up duration of the patients in the navigation group remained short at the time of writing, and several patients showed insufficient muscle recovery, the clinical results will be compared after at least 1 year of follow-up for the patients in the navigation group.
The usefulness of computer navigation in osteotomy includes its ability to perform 3D preoperative planning, enable safe performance of the osteotomy even in the case of a poor visual field, reduce exposure to radiation from intraoperative fluoroscopy (or eliminate the need for intraoperative fluoroscopy), and display the tip position of the chisel in real time, which is educationally beneficial because it also allows staff other than the operator to follow the progress of the surgery. The disadvantages include the necessity of tracker installation and exposure to radiation associated with preoperative CT imaging.
In future investigations, the accuracy of computer navigation in osteotomy should be verified. In THA, the accuracy of CT-based navigation can be evaluated by comparing the angle and position of a placed implant determined by postoperative CT to the values indicated by the navigation during surgery. However, the accuracy is difficult to precisely evaluate in osteotomy without a metal implant; therefore, procedures to evaluate the accuracy for hip osteotomies should be developed in future.
With regard to 3D preoperative planning, since there is no accepted standard for the optimal osteotomy site or the direction and amount of movement, further investigation of ideal preoperative planning is required. In future, the optimal direction and amount of transposition could be decided using motion simulation analysis to avoid femoroacetabular impingement after movement and finite element analysis to obtain sufficient reduction of hip joint contact stress.10) Regarding surgery assistance, the osteotomy site can be confirmed but the position of the bone fragment after osteotomy cannot be tracked. Therefore, a detailed method that confirms the position after bone fragment movement should be developed. Therefore, computer navigation will likely become increasingly useful, although further development of this system is essential.
In conclusion, rotational acetabular osteotomy is a well-established surgical procedure that allows joint preservation, but is technically demanding. A more accurate and safer osteotomy can be performed with 3D preoperative planning and the use of intraoperative computer navigation.
Figures and Tables
Fig. 1
Preoperative planning of rotational acetabular osteotomy using computer software. (A) In the preoperative planning of the osteotomy line, spherical osteotomy of the acetabulum is planned so that the center of the sphere is near the center of the femoral head (or the center of the hip joint). (B) The plan is to rotate it laterally until the acetabular roof obliquity angle becomes 0° and the anterior coverage is corrected.
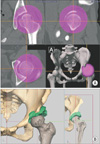
Fig. 2
Marking of the osteotomy line using a registered high-speed drill. (A) The tip position of the high-speed drill can be confirmed on the computer screen during surgery (arrow: the intersection of two orange lines). (B) The osteotomy line is marked with the high-speed drill according to the preoperative plan while the position of the tip of the high-speed drill is confirmed (arrows).
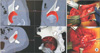
Fig. 3
Osteotomy using a registered curved chisel. (A) The tip position of the curved chisel can be confirmed on the computer screen during surgery (arrow: the intersection of two orange lines). (B) Osteotomy is performed according to the preoperative plan with real-time image guidance.
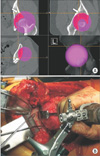
Fig. 4
Confirmation of position after transposition of the bone fragment. (A) By touching the surface of the rotated bone fragment with a pointer after rotating the bone fragment obtained by osteotomy and fixing it with three polylactic acid absorbable screws, it is confirmed whether or not the position fits the surface of the planned position after rotation in the preoperative plan. (B) The arrow shows the pointer tip, which touches the surface of the rotated bone fragment at the same position as in the preoperative plan (yellow portion).
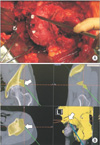
Fig. 5
Case presentation of rotational acetabular osteotomy using a computed tomography (CT)-based navigation. A female patient aged 19 years with bilateral acetabular dysplasia. Rotational acetabular osteotomy of the right hip was performed using a CT-based navigation system. The acetabular head index (AHI) was 68%, center-edge (CE) angle was 10°, and acetabular roof angle was 21° in the right hip preoperatively; these improved to AHI of 92%, CE angle of 40°, and acetabular roof angle of 0° postoperatively. (A) Preoperative frontal radiograph of both hips. (B) Preoperative lateral radiograph of the right hip. (C) Frontal radiograph of both hips at 6 weeks postoperatively. (D) Lateral radiograph of the right hip at 6 weeks postoperatively.
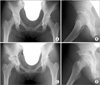
Table 1
Radiographic Measurements in the Navigation and Non-Navigation Groups

ACKNOWLEDGEMENTS
We would like to thank Masatoshi Oba, MD, for his support in editing the video file.
References
1. Ninomiya S, Tagawa H. Rotational acetabular osteotomy for the dysplastic hip. J Bone Joint Surg Am. 1984; 66(3):430–436.

2. Nozawa M, Shitoto K, Matsuda K, Maezawa K, Kurosawa H. Rotational acetabular osteotomy for acetabular dysplasia: a follow-up for more than ten years. J Bone Joint Surg Br. 2002; 84(1):59–65.
3. Takatori Y, Ninomiya S, Nakamura S, et al. Long-term results of rotational acetabular osteotomy in patients with slight narrowing of the joint space on preoperative radiographic findings. J Orthop Sci. 2001; 6(2):137–140.

4. Massie WK, Howorth MB. Congenital dislocation of the hip. Part I: method of grading results. J Bone Joint Surg Am. 1950; 32(3):519–531.
5. Iwana D, Nakamura N, Miki H, Kitada M, Hananouchi T, Sugano N. Accuracy of angle and position of the cup using computed tomography-based navigation systems in total hip arthroplasty. Comput Aided Surg. 2013; 18(5-6):187–194.

6. Akiyama H, Goto K, So K, Nakamura T. Computed tomography-based navigation for curved periacetabular osteotomy. J Orthop Sci. 2010; 15(6):829–833.

7. Langlotz F, Bachler R, Berlemann U, Nolte LP, Ganz R. Computer assistance for pelvic osteotomies. Clin Orthop Relat Res. 1998; (354):92–102.

8. Hsieh PH, Chang YH, Shih CH. Image-guided periacetabular osteotomy: computer-assisted navigation compared with the conventional technique: a randomized study of 36 patients followed for 2 years. Acta Orthop. 2006; 77(4):591–597.

9. Tokunaga K, Watanabe K. Accuracy of the surface-match registration on the inner table of the pelvis in the CT-based navigation for the curved periacetabular osteotomy. Hip Joint. 2012; 38:161–165.
SUPPLEMENTARY MATERIALS
Video clip for surgical procedure of RAO using CT-based navigation. A video clip is available in the electronic version of this paper at the CiOS website, www.ecios.org.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download