Abstract
Background
Epidural hematoma is a rare but serious complication. According to previous studies, it is not prevented by suction drains. This study evaluated the following alternative hypothesis: the larger the diameter of a suction drain, the less the remaining epidural hematoma after spinal surgery.
Methods
This was a randomized prospective study. Patients who underwent posterior lumbar decompression and instrumented fusion were divided into two groups: the large drain (LD, 2.8-mm-diameter tube) and small drain (SD, 1.6-mm-diameter tube) groups according to the diameter of the suction drains. All patients were consecutive and allocated alternately according to the date of operations. Suction drains were removed on day 3 and magnetic resonance imaging was performed on day 7 postoperatively. The size of remaining hematomas was measured by the degree of thecal sac compression in cross section using the following 4-point numeric scale: G1, less than one quarter; G2, between one quarter and half; G3, more than half; and G4, more than subtotal obstruction.
Results
There were 39 patients with LDs and 38 with SDs. They did not differ significantly in terms of sex, number of fusion segments, revision or not, antiplatelet medication, intraoperative injection of tranexamic acid. However, patient age differed significantly between the two groups (LD, 63.3 years and < SD, 68.6 years; p = 0.007). The two groups did not differ significantly in terms of prothrombin time, activated partial thromboplastin time, platelet number, blood loss, or operation duration. However, platelet function analysis exhibited a significant difference (LD, 164.7 seconds and < SD, 222.3 seconds; p = 0.002). The two blinded readers showed high consistency (Kappa value = 0.740; p = 0.000). The results of reader 1 were as follows: LD and SD had 21 and 21 cases of G1, 9 and 11 cases of G2, 6 and 6 cases of G3, and 3 and 0 cases of G4, respectively. The results of reader 2 were as follows: LD and SD had 22 and 23 cases of G1, 7 and 9 cases of G2, 7 and 6 cases of G3, and 3 and 0 cases of G4, respectively. There was no difference between the two groups (reader 1, p = 0.636; reader 2, p = 0.466).
Postoperative spinal epidural hematoma (POSEH) after spinal surgery is a rare but serious complication. The incidence of symptomatic POSEH has been reported to be 0.1% to 0.2%.1234) However, Leonardi et al.5) reported an incidence of 28% in a prospective study. Furthermore, the incidence of asymptomatic cases has been reported to be 33% to 100%.67891011) Determining the incidence of POSEH is problematic due to the marked variability in symptom severity. There is general agreement that a symptomatic hematoma should be evacuated as soon as possible.121314) However, there is no consensus regarding risk factors.2415) Therefore it would be difficult to set up preventive measures based on established evidences. Establishment of a fail-safe measure to avoid such a complication would be more valuable than identifying risk factors, which frequently cannot be avoided. High estimated blood loss does not mean a high risk of epidural hematoma. If suction drains function well, the remaining hematoma will be small. However, several studies have not demonstrated that suction drains can prevent a symptomatic epidural hematoma.121416171819202122) According to the Hagen-Poiseuille law, the volume of a fluid transferred at a functional time is proportional to the biquadrate of the diameter of a tube. Therefore, larger-diameter suction drains may be able to prevent epidural hematoma accumulation. In this study, the following alternative hypothesis, the larger the diameter of suction drains, the smaller the remaining epidural hematoma, was evaluated.
This was a randomized unblinded prospective study, which was approved by the Institutional Review Board of Seoul Sacred Heart General Hospital. Patients who underwent posterior decompression and instrumented fusion of the lumbar spine in the hospital during a 4-month period were enrolled. Informed written consent was received from all subjects. Subjects were divided into the following two groups: the large drain (LD) and small drain (SD) groups. The LD group used two 2.8-mm-diameter tubes that contained 22 holes of 2.2 mm diameter at 10 cm intervals, and the SD group used two 1.6-mm-diameter tubes that contained 32 holes of 1.1 mm diameter at 10 cm intervals (Fig. 1). Each tube was connected to a negative pressure bag (120 ± 30 mmHg; EZ-VAC, EZ Medisys Co., Goyang, Korea). All patients were operated on by the same surgeon, the senior author, and were allocated alternately according to the date of operations. Operations were performed according to the standard practice at the Seoul Sacred Heart General Hospital. Neither gelfoam nor any type of hemostatic material was used. Two drain tubes were placed over the laminectomy site in a parallel fashion as close as possible to the dura mater. Negative vacuum pressure was applied to drains within 8 minutes after the commencement of wound closure in all cases. Drains were removed and ambulation was allowed on day 3. All patients underwent magnetic resonance imaging (MRI) at day 7 postoperatively. To demonstrate the homogeneity of the two groups, data regarding demographics and blood coagulation parameters were compared. Total blood loss and blood loss per 10 minutes were also compared. The magnitude of any remaining hematoma was assessed by the degree of thecal sac compression on T2-weighted axial MRI which showed maximal compression. Two orthopedic surgeons blinded to the current study measured thecal sac compression independently according to the following 4-point scale; G1 less than one quarter, G2 between one quarter and half, G3 more than half, and G4 more than subtotal obstruction (Fig. 2). Differences in remaining epidural hematoma were analyzed by Mann-Whitney U-test. To demonstrate the homogeneity of the two groups, an independent t-test for numeric variables and chi-squared test for ordinal and nominal variables were applied. When expected values were less than 5, a linear-by-linear association was performed. Confidence interval was set as 95%. The SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
There were 39 cases in the LD group and 38 in the SD group. The following demographic parameters did not differ significantly between the LD and SD groups: sex (male/female: 13/26, 12/26), number of fusion segments (1.2, 1.3), virgin or revision operation (primary/revision: 26/6, 19/8), anti-platelet medication (nil/use/stop: 32/5/2, 29/8/1), tranexamic acid injection during the operation (nil/use: 10/29, 16/22). However, patient age differed significantly between the two groups (LD, 63.3 years < SD, 68.6 years; p = 0.007) (Table 1). The following coagulation-related parameters did not exhibit significant differences between the LD and SD groups: prothrombin time (10.1, 10.2 seconds), activated partial thromboplastin time (29.0, 28.6 seconds), and platelet count (275,540, 261,000/mL). However, platelet function analysis differed significantly between the two groups (LD, 164.7 seconds < SD, 222.3 seconds; p = 0.002). There was no significant difference in estimated blood loss (812.8, 859.4 mL), blood loss per 10 minutes (44.0, 46.2 mL) and operation duration (183.6, 185.2 minutes) between the groups (Table 2). The thecal sac compression measurements showed high consistency between the two readers (Kappa value = 0.740, p = 0.000). The results of reader 1 were as follows; LD and SD groups had 21 and 21 cases of G1, 9 and 11 cases of G2, 6 and 6 cases of G3, and 3 and 0 cases of G4, respectively (p = 0.636). The results of reader 2 were as follows: the LD and SD groups had 22 and 23 cases of G1, 7 and 9 cases of G2, 7 and 6 cases of G, and 3 and 0 cases of G4, respectively (p = 0.466). There was no significant difference (Table 3). Three cases of G4 in the LD group had symptoms. None of the patients had neurological symptoms immediately postoperatively and ambulation began as planned. However, a patient complained of leg pain while walking on day 7, a second patient complained of pain in both buttocks and gait disturbance on day 9, and a third patient complained of pain in both legs while walking on day 9 postoperatively. None of these three patients had motor weakness. Hematoma evacuation was performed on days 9, 10, and 10, respectively under local anesthesia. Symptom improvement was assessed at the scene immediately following evacuation. All three patients' symptoms were ameliorated at the scene; no neurological sequelae were detected.
Although the incidence of symptomatic POSEH is low, it is a serious complication. The magnitude and complexity of spinal surgeries is becoming greater and estimated blood loss is increasing. Furthermore, antithrombotic treatment is performed more frequently. Previous studies have focused on the incidence and risk factors; however, there is as yet is no consensus regarding the risk factors for POSEH. According to retrospective case studies, significantly increased risk has been reported in patients over 60 years old, with Rh-positive blood types, intraoperative hemoglobin values < 10 g/dL, and international normalized ratio (INR) values > 2.0 within the first 48 hours postoperatively.1) Multilevel procedures and preoperative coagulopathy significantly increase the risk of symptom development,2) and preoperative nonsteroidal anti-inflammatory drugs and large intraoperative blood loss volumes were reported to be significant predisposing factors.4) In a prospective study in asymptomatic patients, a larger volume of hematoma was associated with advanced age, multilevel procedure, and INR.15) Because symptomatic POSEH develops in 0.1% to 0.2% of cases,1234) performing a randomized prospective study of the risk factors of symptomatic cases is nearly impossible. We still do not know the size of hematoma which can develop symptoms. Awwad and Smith23) reported marked spinal canal compression as a normal finding in the immediate post-laminectomy period. In nine of 10 patients, postoperative thecal sac compression was greater than that of preoperative state. Another study reported that the size of POSEH is an important factor.5) In the prospective study by Sokolowski et al.15) of symptomatic and asymptomatic POSEH, the amount of remaining hematoma and degree of thecal sac compression were not significantly different; however, the critical ratio (i.e., postoperative:preoperative cross-sectional area ratio) was significantly lower in the symptomatic group.
Use of a suction drain facilitates removal of an intrawound hematoma; however, several studies have reported that a suction drain does not prevent the development of such a complication. Only one prospective study has reported that a suction drain influences POSEH. Mirzai et al.24) prospectively randomized 50 patients undergoing single-level lumbar discectomy into two groups, one with drains placed and one without, and performed an MRI on all patients on postoperative day 1. The group without drains developed epidural fluid collection at a significantly higher rate of 89% compared to 36% of those with drains.24) We attempted to determine why suction drains do not prevent POSEH. According to the HagenPoiseuille law, a large-diameter drain tube should suck out remaining blood more effectively than a tube with a smaller diameter. The LD had 89.7 mm2 and the SD 32.4 mm2 areas of pores per 10 cm length; thus the LD had a 2.8-fold greater pore area. The diameter of LD was 1.75-fold greater than that of SD. Therefore, the drainage capacity of LD should be 1.754 = 9.38-fold greater than that of SD. Other than the size of a drain tube, blood viscosity and drain tube position could affect the results. We thought that drain tubes should be placed as close as possible to the dura mater so as not to allow a hematoma mass close to the thecal sac. Moreover, vacuum should be connected before clotting of extravascular blood. Any materials that can activate platelet and facilitate coagulation of extravascular blood were avoided to prevent dysfunction of suction drains. However, there was no difference in the size of remaining hematoma between the two groups. In other words, a SD was sufficient to evacuate the remaining blood. However, symptomatic POSEHs developed in the LD group. Thus other, as-yetunknown factors must be involved in POSEH development.
The evaluation was based on thecal sac compression rather than the actual amount of hematoma. This was because we believed that the amount of hematoma that does not compress the thecal sac is unimportant. Our grading system showed acceptable agreement between the two readers, and the results of each reader were independently significant.
There were three cases of G4, all in the LD group. The reason for this is unclear. None of them was a revision procedure and two involved use of tranexamic acid intraoperatively. Not all were multi-segment cases; two cases were of two segments, and one case of one segment.
None of the three patients complained of leg pain prior to MRI. We wondered whether their leg pain was influenced by the physician's suggestion. The degree of pain would have been considered nonspecific if an MRI had not been performed. We performed a hematoma evacuation under local anesthesia to demonstrate its contribution to the symptoms. The leg pain began to improve immediately after removal of the hematoma in all three patients. We expected a greater number of POSEH cases with mild-to-moderate symptoms. We were unsure whether this type of POSEH would have neurological sequelae or resolve spontaneously. However, many undiscovered cases are likely extant.
There were several limitations to our study. Although it was of a prospective design, the patient allocation was poorly randomized according to date and the operators were not blinded. Moreover, the actual three-dimensional volume of the hematoma was not measured. Therefore, we measured the degree of thecal sac compression. However, to the best of our knowledge, the present study was the first to investigate differences in complications according to the size of suction drain tubes.
In conclusion, there was no difference between the LD and SD groups. The alternative hypothesis was rejected. Therefore, small suction drains are sufficient to prevent hematoma accumulation. However, our results do not indicate why suctions drains frequently cannot prevent POSEH.
Figures and Tables
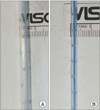 | Fig. 1(A) The large drain: a 2.8-mm-diameter tube with 22 holes of 2.2 mm diameter per 10 cm length. The total area of holes per 10 cm was 89.7 mm2. (B) The small drain: a 1.6-mm-diameter tube with 32 holes of 1.1 mm diameter per 10 cm length. The total area of holes per 10 cm was 32.4 mm2. The large drain has 2.8-fold greater hole area and 1.75-fold greater tube diameter than the small drain. Therefore, the drainage capacity of the large drain is 1.754 : 9.38-fold greater than that of a small drain according to the Hagen-Poiseuille law. |
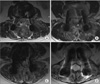 | Fig. 2Grading system of epidural hematoma as determined by thecal sac compression on T2-weighted axial images. (A) G1: less than one quarter. (B) G2: between one quarter and half. (C) G3: more than half. (D) G4: more than subtotal obstruction. |
Table 1
Demographic Characteristics of the Patients

Table 2
Coagulation-Related Data of the Patients

Table 3
Difference in Thecal Sac Compression between the Large Drain and Small Drain Groups

References
1. Awad JN, Kebaish KM, Donigan J, Cohen DB, Kostuik JP. Analysis of the risk factors for the development of postoperative spinal epidural haematoma. J Bone Joint Surg Br. 2005; 87(9):1248–1252.

2. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002; 27(15):1670–1673.

3. Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg. 1995; 83(1):1–7.

4. Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004; 16(6):e1.
5. Leonardi MA, Zanetti M, Saupe N, Min K. Early postoperative MRI in detecting hematoma and dural compression after lumbar spinal decompression: prospective study of asymptomatic patients in comparison to patients requiring surgical revision. Eur Spine J. 2010; 19(12):2216–2222.

6. Ross JS, Masaryk TJ, Modic MT, Bohlman H, Delamater R, Wilber G. Lumbar spine: postoperative assessment with surface-coil MR imaging. Radiology. 1987; 164(3):851–860.

7. Dina TS, Boden SD, Davis DO. Lumbar spine after surgery for herniated disk: imaging findings in the early postoperative period. AJR Am J Roentgenol. 1995; 164(3):665–671.

8. Montaldi S, Fankhauser H, Schnyder P, de Tribolet N. Computed tomography of the postoperative intervertebral disc and lumbar spinal canal: investigation of twenty-five patients after successful operation for lumbar disc herniation. Neurosurgery. 1988; 22(6 Pt 1):1014–1022.

9. Djukic S, Vahlensieck M, Resendes M, Genant HK. The lumbar spine: postoperative magnetic resonance imaging. Bildgebung. 1992; 59(3):136–146.
10. Kotilainen E, Alanen A, Erkintalo M, Helenius H, Valtonen S. Postoperative hematomas after successful lumbar microdiscectomy or percutaneous nucleotomy: a magnetic resonance imaging study. Surg Neurol. 1994; 41(2):98–105.

11. Ikuta K, Tono O, Tanaka T, et al. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: a clinical and magnetic resonance imaging study. J Neurosurg Spine. 2006; 5(5):404–409.

12. Amiri AR, Fouyas IP, Cro S, Casey AT. Postoperative spinal epidural hematoma (SEH): incidence, risk factors, onset, and management. Spine J. 2013; 13(2):134–140.

13. Cabana F, Pointillart V, Vital J, Senegas J. Postoperative compressive spinal epidural hematomas: 15 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000; 86(4):335–345.
14. Yi S, Yoon DH, Kim KN, Kim SH, Shin HC. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J. 2006; 47(3):326–332.

15. Sokolowski MJ, Garvey TA, Perl J 2nd, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine (Phila Pa 1976). 2008; 33(1):108–113.
16. Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine (Phila Pa 1976). 2004; 29(10):1066–1068.

17. Chimenti P, Molinari R. Post-operative spinal epidural hematoma causing American Spinal Injury Association B spinal cord injury in patients with suction wound drains. J Spinal Cord Med. 2013; 36(3):213–219.

18. Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007; (3):CD001825.

19. Walid MS, Abbara M, Tolaymat A, et al. The role of drains in lumbar spine fusion. World Neurosurg. 2012; 77(3-4):564–568.

20. Kanayama M, Oha F, Togawa D, Shigenobu K, Hashimoto T. Is closed-suction drainage necessary for single-level lumbar decompression?: review of 560 cases. Clin Orthop Relat Res. 2010; 468(10):2690–2694.

21. Uribe J, Moza K, Jimenez O, Green B, Levi AD. Delayed postoperative spinal epidural hematomas. Spine J. 2003; 3(2):125–129.

22. Scuderi GJ, Brusovanik GV, Fitzhenry LN, Vaccaro AR. Is wound drainage necessary after lumbar spinal fusion surgery? Med Sci Monit. 2005; 11(2):CR64–CR66.
23. Awwad EE, Smith KR Jr. MRI of marked dural sac compression by surgicel in the immediately postoperative period after uncomplicated lumbar laminectomy. J Comput Assist Tomogr. 1999; 23(6):969–975.

24. Mirzai H, Eminoglu M, Orguc S. Are drains useful for lumbar disc surgery? A prospective, randomized clinical study. J Spinal Disord Tech. 2006; 19(3):171–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download