Abstract
Background
Postoperative spinal epidural hematoma (POSEH) is different from spontaneous or post-spinal procedure hematoma because of the application of suction drains. However, it appeared that suction drains were not effective for prevention of POSEH in previous studies. The purpose of this study was to test our hypothesis that POSEH can be caused by hypercoagulability.
Methods
This was an experimental study. One hundred fifty milliliters of blood was donated from each of the 12 consecutive patients who underwent spine surgery and infused into 3 saline bags of 50 mL each. One of the 3 bags in each set contained 5,000 units of thrombin. All of them were connected to 120 ± 30 mmHg vacuum suctions: drainage was started 8 minutes after connection to the vacuum system for 12 normal blood bags (BV8) and 12 thrombin-containing blood bags (TBV8) and 15 minutes after connection for the remaining 12 normal blood bags (BV15). The amount of initial and remaining hematoma at 20 minutes, 120 minutes, and 24 hours after vacuum application were measured by their weight (g). The primary endpoint was the difference between BV8 and TBV8. The secondary end point was the difference between BV8 and BV15.
Results
The remaining hematoma in TBV8 was significantly greater than that in BV8 at all measurement points: 46.3 ± 12.4 vs. 17.0 ± 1.3 (p = 0.000) at 20 minutes; 33.0 ± 8.2 vs. 16.3 ± 1.2 (p = 0.000) at 120 minutes; and 26.1 ± 4.0 vs. 15.8 ± 1.6 (p = 0.000) at 24 hours after vacuum application. The remaining hematoma of BV15 was significantly greater than that of BV8 at all measurement points: 30.0 ± 12.0 vs. 17.0 ± 1.3 (p = 0.002) at 20 minutes; 24.2 ± 7.6 vs. 16.3 ± 1.2 at 120 minutes (p = 0.002); and 22.2 ± 6.6 vs. 15.8 ± 1.6 (p = 0.004) at 24 hours after vacuum application.
Conclusions
With a suction drain in place, the amount of remaining hematoma could be affected by coagulability. Thrombin-containing local hemostatics and the length of time elapsed before the commencement of suction resulted in hypercoagulability, indicating these two factors could be causes of POSEH.
Postoperative spinal epidural hematoma (POSEH) is not common but can lead to serious neurological sequelae. The magnitude of dural compression sufficient to induce symptoms of epidural hematomas has not been clearly elucidated. Though there is a wide spectrum of characteristics of POSEH, it is largely divided into two types depending on the presence of symptoms (symptomatic and asymptomatic). Many surgeons are reluctant to use antiplatelet drugs perioperatively under the assumption that bleeding tendency and hypocoagulability would increase the risk of POSEH.1) Most previous studies on risk factors for spinal epidural hematomas have focused on the bleeding tendency; however, they have failed to demonstrate it as a risk factor at least for POSEH.2) POSEHs are considered different from spontaneous and post-epidural catheterization hematomas because of the application of suction drains in most spine surgeries. More bleeding does not necessarily result in a greater volume of remaining hematoma if a suction drain functions well. Careful and meticulous hemostasis is different from hypercoagulability of extra-vascular blood. In our experience, POSEH occurred more often when local hemostatics were used. Thus, we presumed that hypercoagulability of extra-vascular blood induced by local hemostatic agents or a prolonged delay in connection to the vacuum system would inhibit appropriate function of suction drains, increasing the volume of remaining hematoma. In this study, we hypothesized that if coagulability is increased by local hemostatic agents or if extravascular blood is clotted more by a delay in drainage, the amount of remaining hematoma would increase. To test our hypothesis, we conducted an experiment using models that seemed similar to a postoperative epidural incisional space.
This was an experimental study using the blood of 12 patients who underwent spine surgery in July 2015. Written informed consents were received from all patients who willingly donated their blood for the current study conducted with approval of the Institutional Review Board of Seoul Sacred Heart General Hospital. Patients who were under 60 years of age and over 80 years of age were excluded. Patient demographics and blood coagulation-related laboratory data were investigated. A 1.6-mm diameter drain tube (Ez-VAC; EZ Medisys, Goyang, Korea) was inserted into a portal of a 100 mL empty plastic saline bag with two portals. The drain tube had 26 holes of 1.1-mm diameter at the most distal portion. The gap around the drain was filled with silicone gel. Three sets of these bags were prepared for each case. One of them was filled with 5.6 mg of crushed gelfoam (Cutanplast; Mascia Brunelli, Milano, Italy) moistened with 5,000 units of porcine thrombin (Reyon pharmaceutical Co., Jincheon, Korea). The drain was connected to a vacuum bag (Ez-VAC; EZ Medisys) at 120 ± 30 mmHg. One hundred and fifty milliliters of blood was harvested from each of the 12 consecutive patients who underwent spinal surgery. Fifty milliliters of blood was injected into each saline bag. For one normal blood filled bag and one thrombin added blood bag, vacuum drainage was started 8 minutes after connection to the vacuum system (BV8 and TBV8). For the remaining one normal blood filled bag, the vacuum pump was turned on 15 minutes after connection (BV15). The amount of initial and remaining hematoma at 20 minutes, 120 minutes, and 24 hours were assessed by weight (Fig. 1). For TBV8, the weight of thrombin and gelfoam was subtracted from the weight measured after drainage. The same procedure was done for all samples obtained from 12 patients (Fig. 2). The primary end point was the difference of remaining hematoma between BV8 and TBV8 to investigate the influence of hypercoagulability induced by local hemostatic agents on the proper function of a suction drain. The secondary end point was the difference of remaining hematoma between BV8 and BV15 to investigate the influence of a delay in drainage on the proper function of a suction drain. Statistical analysis was conducted using an independent t-test. The SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. The confidence interval was set as 95%. Power was calculated using a post-hoc power calculator of ClinCalc (http://clincalc.com/).
There were 12 consecutive patients who donated their blood and met the physical criteria. There were 1 male and 11 females. Their mean age was 68.3 years (range, 56 to 78 years). Demographics and coagulation-related data are tabulated in Table 1.
Data comparing BV8 and TBV8 are presented in Table 2. The initial weight was not significantly different, but the amount of remaining hematoma of TBV8 was significantly greater than that of BV8 at 20 minutes, 120 minutes, and 24 hours after initiation of drainage. Statistical power was 100% at all measurement times. The standard deviations of TBV8 were greater than those of BV8 at each time.
Data comparing BV8 and BV15 are shown in Table 3. The amount of remaining hematoma of BV15 was significantly greater than that of BV8 at 20 minutes, 120 minutes and 24 hours after initiation of drainage. Statistical power was over 90% at all measurements. Standard deviations of BV15 were greater than those of BV8 at each time.
Risk factors for POSEH are mostly theoretical and sometimes presumptive. Therefore, avoiding the known risk factors is not a reliable strategy to prevent POSEH. Suction drainage can be expected to help obviate the complication in case of certain amount of intraoperative bleeding; however, a multitude of previous studies have shown that it is not a fail-safe way to prevent the catastrophic complication.3456789101112) There was only one prospective study that demonstrated the efficacy of suction drainage in reduction of remaining epidural hematoma.13) Therefore, we conjectured there must be some factors that hamper appropriate function of suction drains, which should also be recognized as the risk factors for POSEH.
There have been many retrospective studies suggesting the risk factors for POSEH. Old age, nonsteroidal anti-inflammatory drugs, large intraoperative blood loss, intraoperative hemoglobin of less than 10 g/dL, Rh+ blood type, and international normalized ratio (INR) of more than 2.0 have been considered attributable to the development of POSEH.3714) Sokolowski et al.15) reported that old age, multilevel procedure, and high INR were associated with large volume of POSEH in their prospective study, but they did not take consideration into the presence of symptoms. Antiplatelet drugs have been regarded to increase the risk of POSEH and most spinal surgeons have been reluctant to operate on patients taking them.1) However, a more recent study by Cuellar et al.2) has reported there was no appreciable increase in bleeding-related complication rates in patients with cardiac stents undergoing spine surgeries while continuing to take aspirin compared with patients who discontinued aspirin prior to surgery. On the contrary, there was a case report suggesting hypercoagulability as a risk factor.16) Thus, the causal relationship between POSEH and hypocoagulability is inconclusive yet.
Based on previous research, we presumed the conditions that can lead to the occurrence of POSEH include massive bleeding, the presence of a dead space enough to allow large volume of coagulated hematoma, and early coagulation of the extravascular blood that overwhelms suction drains.
Though the limited efficacy of suction drainage in prevention of POSEH has been documented in many studies, little research has been undertaken to elucidate the reason. Coagulopathy, antiplatelet drugs, and thrombolytic agents increase intraoperative bleeding and thus may be risk factors for a spontaneous spinal epidural hematoma.171819) At the same time, unclotted blood would be easily drained through a suction drain. Watery state fluid usually does not compress the thecal sac in the open space exposed by laminectomy. However, if the fluid forms a solid material, it can create enough pressure to compress the thecal sac.
In our experiment, hypercoagulability disrupted appropriate function of the suction drain causing more hematoma to remain. As the elapsed time increased, the extravascular blood surely clotted more. Our data suggest that the vacuum should be connected as early as possible although a fail-safe time limit was not investigated yet. The real situation would be different from the current model because there would be continuous bleeding from the wound and epidural vessels. If the extravascular blood is not evacuated rapidly and forms a clotted mass, it would act as a nidus for further clotting. The standard deviations of BV15 were bigger than those of BV8, which suggests that the remaining blood cannot be drained uniformly over time.
Thrombin-containing local hemostatics (TCLH) have been used for a long time. To our knowledge, there have been no reports associating the use of TCLH with the risk of epidural hematoma although it has been advised to remove the remnants of the agents for a precautionary measure. Based on our experiment, TCLH should be removed upon completion of hemostasis and before initiation of wound closure. Using TCLH just before wound closure can be considered more dangerous.
Given the statistical power (> 90%), the sample size was sufficient to test the hypotheses. However, there were several limitations to this study. We could not use the same sample for all experiments. Because we used real patients' blood, it was difficult to harvest more than 150 mL of blood from a patient. However, their age and coagulationrelated factors were analyzed to ensure homogeneity. Though one set of experiment used the blood of the same patient, the initial weight was not identical. The error happened because the amount of blood and sealing silicon for each saline bag was slightly different. This was the reason why we applied the Student t-test rather than a paired t-test.
In conclusions, coagulation of extravascular blood inhibited effective function of suction drains and resulted in a large amount of remaining hematoma. It appeared that this could be accelerated by TCLH and a delay in activation of suction drainage. We believe that if a suction drain functions well before a coagulation nidus is formed, POSEH could be prevented.
Figures and Tables
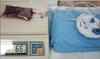 | Fig. 1Photograph of the experimental setup. The blood filled bag was connected to a 120 ± 30 mmHg vacuum system through a 1.6-mm diameter drain tube and the weight was measured. |
 | Fig. 2Flowchart of the experiment. Blood obtained from a patient was divided into 3 bags. Two of them had normal blood and the other had thrombin (Th) added blood. Twelve normal and 12 Th added blood bags were connected to the vacuum and drainage was initiated 8 minutes after connection. The other 12 normal blood bags were connected to the vacuum and drainage was initiated 15 minutes after connection. The amount of remaining hematoma was measured at 20 minutes, 120 minutes, and 2 hours after initiation of drainage. |
Table 1
Demographics and Coagulation Related Lab Findings
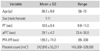
Table 2
Weight of Remaining Hematoma (BV8:TBV8)

| Variable | Initial | 20 Min | 120 Min | 24 Hr |
|---|---|---|---|---|
| BV8 (g) | 66.8 ± 2.5 | 17.0 ± 1.3 | 16.3 ± 1.2 | 15.8 ± 1.6 |
| TBV8 (g) | 67.3 ± 2.1 | 46.3 ± 12.4 | 33.0 ± 8.2 | 26.1 ± 4.0 |
| p-value | 0.301 | 0 | 0 | 0 |
| Statistical power (%) | - | 100 | 100 | 100 |
Table 3
Weight of Remaining Hematoma (BV8:BV15)

References
1. Korinth MC, Gilsbach JM, Weinzierl MR. Low-dose aspirin before spinal surgery: results of a survey among neurosurgeons in Germany. Eur Spine J. 2007; 16(3):365–372.

2. Cuellar JM, Petrizzo A, Vaswani R, Goldstein JA, Bendo JA. Does aspirin administration increase perioperative morbidity in patients with cardiac stents undergoing spinal surgery? Spine (Phila Pa 1976). 2015; 40(9):629–635.

3. Awad JN, Kebaish KM, Donigan J, Cohen DB, Kostuik JP. Analysis of the risk factors for the development of postoperative spinal epidural haematoma. J Bone Joint Surg Br. 2005; 87(9):1248–1252.

4. Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine (Phila Pa 1976). 2004; 29(10):1066–1068.

5. Cabana F, Pointillart V, Vital J, Senegas J. Postoperative compressive spinal epidural hematomas: 15 cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2000; 86(4):335–345.
6. Chimenti P, Molinari R. Post-operative spinal epidural hematoma causing American Spinal Injury Association B spinal cord injury in patients with suction wound drains. J Spinal Cord Med. 2013; 36(3):213–219.

7. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002; 27(15):1670–1673.

8. Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007; (3):CD001825.

9. Scuderi GJ, Brusovanik GV, Fitzhenry LN, Vaccaro AR. Is wound drainage necessary after lumbar spinal fusion surgery? Med Sci Monit. 2005; 11(2):CR64–CR66.
10. Uribe J, Moza K, Jimenez O, Green B, Levi AD. Delayed postoperative spinal epidural hematomas. Spine J. 2003; 3(2):125–129.

11. Walid MS, Abbara M, Tolaymat A, et al. The role of drains in lumbar spine fusion. World Neurosurg. 2012; 77(3-4):564–568.

12. Yi S, Yoon DH, Kim KN, Kim SH, Shin HC. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J. 2006; 47(3):326–332.

13. Mirzai H, Eminoglu M, Orguc S. Are drains useful for lumbar disc surgery? A prospective, randomized clinical study. J Spinal Disord Tech. 2006; 19(3):171–177.
14. Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004; 16(6):e1.
15. Sokolowski MJ, Garvey TA, Perl J 2nd, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine (Phila Pa 1976). 2008; 33(1):108–113.
16. Emori M, Takebayashi T, Imoto K, Ueno S, Mizuno S, Yamashita T. Spinal surgery in a patient with essential thrombocythemia resulting in leg paraplegia: a case report. Spine J. 2013; 13(11):e7–e10.
17. Ahn DK, Jung WS, Lee JI. Hemophilia A in a senior patient: a case report of spinal epidural hematoma as first presentation. Asian Spine J. 2015; 9(3):452–455.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download