Abstract
Background
Laminoplasty is a surgical procedure frequently performed for cervical myelopathy. We investigated correlations between changes in the anteroposterior diameter (APD) of the spinal canal, spinal canal area (SCA), and laminar angle (LA) and clinical outcomes of laminoplasty.
Methods
Of the 204 cervical myelopathy patients who underwent laminoplasty from July 2010 to May 2015, 49 patients who were evaluated with pre- and postoperative computed tomography of the cervical vertebrae were included. The average age of the patients was 60.4 years (range, 31 to 82 years), and the average duration of follow-up was 31.6 months (range, 9 to 68 months). Changes in the APD and SCA were measured at the middle of the vertebral body. Changes in LA were measured where both pedicles were clearly visible. Clinical outcomes were assessed using the Japanese Orthopaedic Association (JOA) score and visual analog scale score for pain preoperatively (1 day before surgery) and postoperatively (last outpatient visit) and examining postoperative complications.
Results
The APD showed an average of 54.7% increase from 11.5 to 17.8 mm. The SCA showed an average of 57.7% increase from 225.9 to 356.3 mm2. The LA increased from 34.2° preoperatively to 71.9° postoperatively. The JOA score increased from an average of 9.1 preoperatively to 13.4 postoperatively. Three patients were found to have hinge fractures during surgery. Postoperative complications, including two cases of C5 palsy, were recorded. The correlation coefficient between the LA change and JOA score improvement was −0.449 (p < 0.05). Patients with a < 33° (25%) increase in the LA showed the most significant clinical improvement.
Radiographic evidence of cervical spondylosis can be observed in > 80% of individuals aged > 60 years.1) Because of the high prevalence, research has been focused on the development of surgical methods to address this disorder. Among them, expansive open-door cervical laminoplasty (EOLP) has been performed in patients with multi-segmental cervical spinal canal stenosis2) with good clinical results.3)
EOLP is advantageous over laminectomy and fusion in terms of the preservation of cervical movement and posterior structures and reduction of adjacent segment disease.456) However, disadvantages of the technique include posterior neck pain, C5 nerve root palsy, and laminar reclosure after surgery.789101112) Many techniques have been developed to prevent postoperative reclosure.13)
O'Brien et al.14) proposed a new laminoplasty technique using a titanium miniplate for prevention of reclosure: its advantages include preservation of the cervical alignment and range of motion and reduction of the reclosure rate and postoperative neck pain.15161718)
Although EOLP using titanium miniplates have been studied in many research, they are limited to clinical and radiological evaluations based only on radiography. In this study, we investigated correlations between radiological and clinical outcomes using computed tomography (CT). The anteroposterior diameter (APD) of the spinal canal, spinal canal area (SCA), and laminar angle (LA) were assessed on CT scans preoperatively and postoperatively (changes in APD, SCA, and LA = ΔAPD, ΔSCA, and ΔLA, respectively). Our hypotheses were that radiological and clinical outcomes would have positive correlations and that patients with optimal ΔSCA, ΔAPD, and ΔLA would show improved clinical outcomes.
Of the 204 cervical myelopathy patients who underwent laminoplasty from July 2010 to May 2015, 49 patients who had preoperative and postoperative CT scans of the cervical vertebrae were included in this study. The inclusion criteria were patients with (1) cervical stenosis between the levels of C3–C7, (2) laminoplasty using a titanium miniplate, and (3) preoperative and postoperative sagittal CT images showing that the bottom surface of each vertebral body was in parallel position (Fig. 1). The exclusion criteria were (1) segmental instability or kyphosis, (2) a history of revision surgery, and (3) a tumor or injury.
Under general anesthesia, the patient was placed in the prone position and the head was slightly flexed using pins. A midline incision was performed to expose the laminae and spinous processes while preserving soft tissue attachments of lateral masses. The spinous processes were then removed. A high-speed drill was used to make a gutter opening on the side of the lamina, just medial to the lateral mass. The surgeon (JSA) drilled the lamina and left a very thin cortex comprising only the ligamentum flavum. The thin lamina was then removed using a 1- or 2-mm Kerrison punch. A hinge-side lamina gutter was made using the same drill, but the inside cortex was not removed. The surgeon gently lifted the laminae with a Penfield dissector, while an assistant held the lamina using a Kocher device to prevent reclosing. The surgeon fixed the opening side using a titanium miniplate (double-bent 12-mm plate; Arch Laminoplasty System, Synthes, West Chester, PA, USA), two 6-mm screws to the laminae, and two 7-mm screws to the lateral mass. Additionally, a foraminotomy was performed in patients with severe radiculopathic symptoms after the laminoplasty.
A hard neck collar was applied temporarily after surgery. The neck collar was removed 8 hours after surgery, and the patients were allowed to stand up and walk. Isometric exercise of the cervical vertebrae was started 1 day after surgery within each patient's pain tolerance.
The medical records and radiological images of the 49 patients were reviewed retrospectively. For accurate radiological assessment, we ensured that each cross-section of the CT image was parallel to the lower surface of the corresponding vertebral body (Fig. 1).
The APD of the spinal canal was measured in the axial plane at the middle of the vertebral body on the sagittal image pre- and postoperatively (Fig. 2A, B, D, E). The SCA was measured in the same plane pre- and postoperatively (Fig. 2A, C, D, F). The LA was measured where both pedicles were clearly visible. The LA was measured between two lines: one line just posterior to the two transverse foramens and the other line on the inner cortex of the lamina on the hinge side. Changes in the LA between preoperative and postoperative measurements were recorded (Fig. 3). All radiographic evaluations were performed using a PACs system (M view; Infinitt, Seoul, Korea). All measurements were performed twice by one of the authors and an independent experienced musculoskeletal radiologist to reduce intraand interobserver variability. All radiological measurements were performed using CT scans (Definition Flash; Siemens Healthcare, Forchheim, Germany) obtained with a 3 mm interval.
Clinical evaluations were performed preoperatively and at the final follow-up. The Japanese Orthopaedic Association (JOA) score was measured to assess clinical function. The JOA recovery rate was calculated with the following formula:19)
A visual analog scale (VAS) was used to assess pain.
Hinge fracture, C5 palsy, and other complications were recorded.
Statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Co., Armonk, NY, USA). The average measurement value of each patient's spinal segment was used for statistical analysis. Pearson correlation analysis was used to assess correlations among the ΔAPD, ΔSCA, ΔLA, and JOA recovery rate. One-way ANOVA was used to determine the optimal ΔLA for excellent clinical outcomes.
The total number of patients who underwent cervical laminoplasty was 49 (34 males and 15 females). The average age of the patients was 60.4 years (range, 31 to 82 years), and the average duration of follow-up was 31.6 months (range, 9 to 68 months).
Table 1 shows pre- and postoperative measurement data. The mean APD showed a 54.7% (range, 10.0% to 120.0%) increase from 11.5 mm (range, 6.5 to 16.5 mm) preoperatively to 17.8 mm (range, 12.0 to 24.9 mm) postoperatively. The mean SCA showed a 57.7% (range, 11.7% to 145.0%) increase from 225.9 mm2 (range, 134 to 325 mm2) preoperatively to 356.3 mm2 (range, 224 to 533 mm2) postoperatively. The mean LA increased by 110% (range, 31% to 224%) from 34.2° (range, 23.4° to 47.5°) preoperatively to 71.9° (range, 46.0° to 85.7°) postoperatively.
Table 2 shows the mean pre- and postoperative SCA of each level. The greatest ΔSCA was observed at the level of C6 (66.05%).
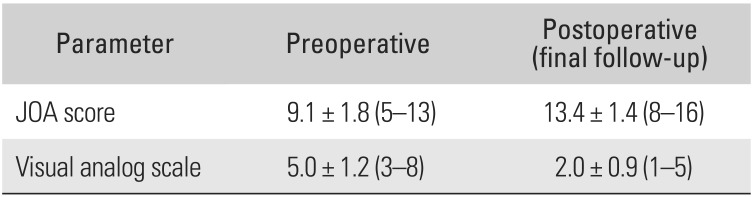
All patients showed clinical improvement. The mean JOA score improved by 4.24 (range, 2 to 7) from 9.1 (range, 5 to 13) preoperatively to 13.4 (range, 8 to 16) at the final follow-up. The mean VAS score for pain improved from 5 (range, 3 to 8) preoperatively to 2 (range, 1 to 5) at the final follow-up (Table 3). The average JOA recovery rate was 54.9 (range, 25.0 to 83.3).
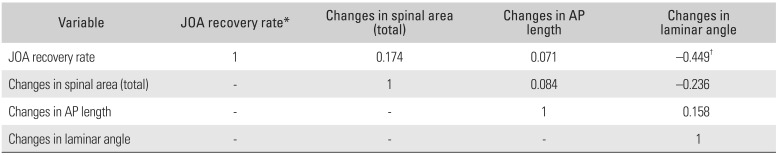
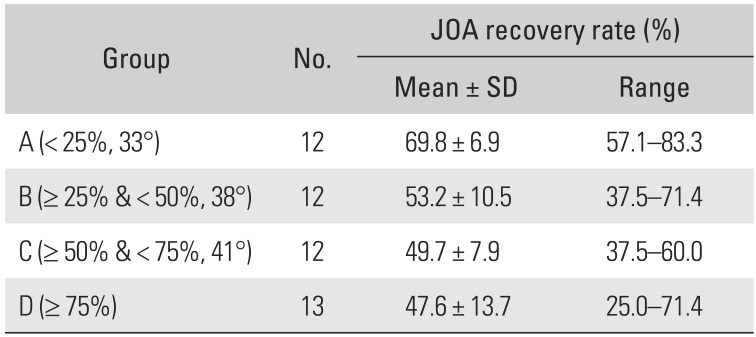
The ΔAPD, ΔSCA, ΔLA, and JOA recovery rates showed normal distributions. A Pearson correlation analysis was performed to determine correlations among the ΔAPD, ΔSCA, ΔLA, and JOA recovery rate (Table 4). A significant correlation (p = −0.449) was observed between ΔLA and JOA recovery rate. However, no other significant correlation was observed between the other variables. One-way ANOVA was used to determine the optimal range of LA. The patients were divided into four groups according to the percentile of ΔLA (group A, ΔLA < 25%; group B, 25% ≤ ΔLA < 50%; group C, 50% ≤ ΔLA < 75%; and group D, ΔLA ≥ 75%). The cutoff values for quartiles were 33° (25%), 38° (50%), and 41° (75%). The clinical outcomes of group A were significantly better than those of the other groups (p < 0.05) (Table 5 and Fig. 4).
A perioperative hinge fracture was noted in 3 of 49 patients (1 case each in groups B, C, and D), and internal fixation using a titanium miniplate was performed immediately. Two cases of left-sided C5 palsy were found in group B; both improved without complications by 3 months after surgery. C5 palsy did not occur in group A. No patient developed reclosure.
The increasing incidence of cervical myelopathy has led to the development and interest of EOLP technique. Since laminoplasty techniques were initially associated with postoperative reclosure, research has been focused on prevention of this complication.13) As part of this effort, O'Brien et al.14) suggested a laminoplasty technique using a titanium miniplate. In this study, we attempted to review the results of the technique performed in our patients to investigate the correlation between clinical and radiological outcomes.
The incidence of cervical myelopathy is increasing as the elderly population continues to grow.20) Laminoplasty, a surgical method developed in Japan, is regarded as the first surgical treatment of choice for cervical myelopathy in Japan.13) An early form of laminoplasty was Z-plasty described by Oyama et al.21) Later, Tsuji22) proposed the en bloc laminotomy as a new method, which Hirabayashi et al.23) further developed into the open-approach laminoplasty. However, the technique had the problem of maintaining the opening state. Hirabayashi and Sotomi24) suggested a suture technique to overcome this, but it resulted in another problem, reclosure of the lamina after surgery. Many surgical techniques, such as the use of bone transplants, hydroxyapatite, ceramic materials, and titanium miniplates, have been developed to address the issue of postoperative reclosure.13)
In this study, a hardware-augmented Hirabayashi-type laminoplasty using titanium miniplates (double-bent 12-mm plate; Arch Laminoplasty System), suggested by O'Brien et al.,14) was performed in all patients to prevent postoperative reclosure, preserve cervical alignment and range of motion, and reduce postoperative neck pain.
Maezumi25) suggested that the LA should be kept to a minimum for desirable clinical outcomes of laminoplasty. In a study by Zhang et al.,26) although no significant difference was found in clinical outcomes between two groups divided according to the ΔLA (15°–30° vs. ≥ 30°), C5 palsy and axial symptoms were less frequent in the group with a ΔLA of 15°–30°. In the present study, two cases of transient C5 palsy were observed in the group with a ΔLA of 25% to 50%. Zhang et al.26) reported that the group with a ΔLA of 15°–30° had a high reclosure rate. However, there was no case of reclosure in our study population.
Ogino et al.27) stated that the ratio of the sagittal diameter to the transverse diameter of the spinal cord was related to the severity of neurological symptoms, and suggested that a decrease in the spinal canal's sagittal diameter (APD) was the most important factor. However, we found no correlation between the APD and JOA recovery rate in the present study.
The most significant finding of our study was the correlation between radiological parameters and the clinical outcome of laminoplasty. Our analyses showed that clinical outcome had a significant correlation with the ΔLA, but not with the ΔSCA or ΔAPD. There was no significant correlation between the ΔLA and ΔSCA because of the inverse relationship of the ΔLA between the hinge side and the opening side.
The LA was different in each patient, although the same metal plate was used in all patients. This might be attributable to minor differences in the position of the fixating screws and gutters. In this study, two cases of left-sided C5 palsy were reported in the group with a ΔLA of 25% to 50%. This may have been due to the surgical site being on the right side, with movement of the spinal cord relatively close to the posterior right side as the lamina opens up unintentionally after surgery, thereby causing radicular paralysis on the left side.
The average increase in the LA ranged from 24.4° to 46.7°. The patients were divided into four groups based on the ΔLA percentile (group A, ΔLA < 25%; group B, 25% ≤ ΔLA < 50%; group C, 50% ≤ ΔLA < 75%; and group D, ΔLA ≥ 75%), and clinical outcomes were compared among the groups. Group A showed the best clinical outcomes; in these patients, there was no need to create a wider angle to achieve a better clinical outcome.
Satomi et al.28) reported that the most favorable JOA score was achieved 3 years postoperatively and that this JOA score was maintained for about 5 years. Thus, if preoperative and postoperative CT scans are taken, we may be able to predict patients' clinical outcomes by calculating the ΔLA. To obtain more accurate CT images, we ensured that each cross-section of the CT images was parallel to the lower surface of the corresponding vertebral body (Fig. 1).
Our study has several limitations. First, the number of patients was not large enough to generate definitive results; however, the same plate (12 mm in length) was used in all patients for laminoplasty. Second, this was not a prospective comparative study; nevertheless, our study did present some significant findings.
In conclusion, laminoplasty with a titanium miniplate is a surgical procedure with well-documented clinical improvement results in patients with cervical myelopathy. Our hypothesis that radiological and clinical outcomes have a positive correlation was partially demonstrated. In particular, a ΔLA of < 33.8° (25%) was associated with better clinical results. However, further studies of laminoplasties using titanium miniplates of different sizes (e.g., 8-mm plates) are needed.
References
1. Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998; 80(1):19–24. PMID: 9460946.

2. Suzuki A, Tamai K, Terai H, et al. Clinical outcome of cervical laminoplasty and postoperative radiological change for cervical myelopathy with degenerative spondylolisthesis. Spine (Phila Pa 1976). 2016; 5. 20. [Epub]. DOI: 10.1097/BRS.0000000000001706.

3. Ahn JS, Lee JK, Lee WW, Hwang JM. Changes in cervical spine range of motion after laminoplasty in cervical spondylotic myelopathy. J Korean Soc Spine Surg. 2012; 19(3):85–89.

4. Chen H, Liu H, Zou L, et al. Effect of Mini-plate Fixation on hinge fracture and bony fusion in unilateral open-door cervical expansive laminoplasty. Clin Spine Surg. 2016; 29(6):E288–E295. PMID: 25023712.

5. Yeh KT, Yu TC, Chen IH, et al. Expansive open-door laminoplasty secured with titanium miniplates is a good surgical method for multiple-level cervical stenosis. J Orthop Surg Res. 2014; 9:49. PMID: 25142174.

6. Lee DH, Park SA, Kim NH, et al. Laminar closure after classic Hirabayashi open-door laminoplasty. Spine (Phila Pa 1976). 2011; 36(25):E1634–E1640. PMID: 21336233.

7. Cho CB, Chough CK, Oh JY, Park HK, Lee KJ, Rha HK. Axial neck pain after cervical laminoplasty. J Korean Neurosurg Soc. 2010; 47(2):107–111. PMID: 20224708.

8. Hosono N, Sakaura H, Mukai Y, Fujii R, Yoshikawa H. C3-6 laminoplasty takes over C3-7 laminoplasty with significantly lower incidence of axial neck pain. Eur Spine J. 2006; 15(9):1375–1379. PMID: 16547754.

9. Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976). 2003; 28(21):2447–2451. PMID: 14595162.
10. Imagama S, Matsuyama Y, Yukawa Y, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br. 2010; 92(3):393–400. PMID: 20190311.
11. Matsumoto M, Watanabe K, Tsuji T, et al. Risk factors for closure of lamina after open-door laminoplasty. J Neurosurg Spine. 2008; 9(6):530–537. PMID: 19035744.

12. Wang HQ, Mak KC, Samartzis D, et al. "Spring-back" closure associated with open-door cervical laminoplasty. Spine J. 2011; 11(9):832–838. PMID: 21890423.

13. Kurokawa R, Kim P. Cervical laminoplasty: the history and the future. Neurol Med Chir (Tokyo). 2015; 55(7):529–539. PMID: 26119898.

14. O'Brien MF, Peterson D, Casey AT, Crockard HA. A novel technique for laminoplasty augmentation of spinal canal area using titanium miniplate stabilization: a computerized morphometric analysis. Spine (Phila Pa 1976). 1996; 21(4):474–483. PMID: 8658252.
15. Rhee JM, Register B, Hamasaki T, Franklin B. Plate-only open door laminoplasty maintains stable spinal canal expansion with high rates of hinge union and no plate failures. Spine (Phila Pa 1976). 2011; 36(1):9–14. PMID: 21192219.

16. Yang HL, Chen GD, Zhang HT, Wang L, Luo ZP. Opendoor laminoplasty with plate fixation at alternating levels for treatment of multilevel degenerative cervical disease. J Spinal Disord Tech. 2013; 26(1):E13–E18. PMID: 23075860.

17. Jiang L, Chen W, Chen Q, Xu K, Wu Q, Li F. Clinical application of a new plate fixation system in open-door laminoplasty. Orthopedics. 2012; 35(2):e225–e231. PMID: 22310411.

18. Chen G, Luo Z, Nalajala B, Liu T, Yang H. Expansive opendoor laminoplasty with titanium miniplate versus sutures. Orthopedics. 2012; 35(4):e543–e548. PMID: 22495857.

19. Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). 2001; 26(17):1890–1894. PMID: 11568701.

20. Fehlings MG, Tetreault L, Nater A, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015; 77(Suppl 4):S1–S5. PMID: 26378347.
21. Oyama M, Hattori S, Moriwaki N, Nitta S. A new method of cervical laminectomy. Chubu Nippon Seikeigeka Gakkai Zasshi. 1973; 16:792–794.
22. Tsuji H. Laminoplasty for patients with compressive myelopathy due to so-called spinal canal stenosis in cervical and thoracic regions. Spine (Phila Pa 1976). 1982; 7(1):28–34. PMID: 7071659.

23. Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976). 1983; 8(7):693–699. PMID: 6420895.

24. Hirabayashi K, Sotomi K. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine State Art Rev. 1987; 1(5):517–532.

25. Maezumi H. Cervical radiculopathy after the posterior decompression of the cervical cord. Ital J Orthop Traumatol. 1989; 20:324–328.
26. Zhang H, Lu S, Sun T, Yadav SK. Effect of lamina open angles in expansion open-door laminoplasty on the clinical results in treating cervical spondylotic myelopathy. J Spinal Disord Tech. 2015; 28(3):89–94. PMID: 22832551.

27. Ogino H, Tada K, Okada K, et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976). 1983; 8(1):1–15. PMID: 6867846.

28. Satomi K, Nishu Y, Kohno T, Hirabayashi K. Long-term follow-up studies of open-door expansive laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976). 1994; 19(5):507–510. PMID: 8184342.

Fig. 1
Postoperative computed tomography (CT) of a 61-year-old male patient who underwent cervical laminoplasty. Each cross-section of the CT image is parallel to the lower surface of the corresponding vertebral body.
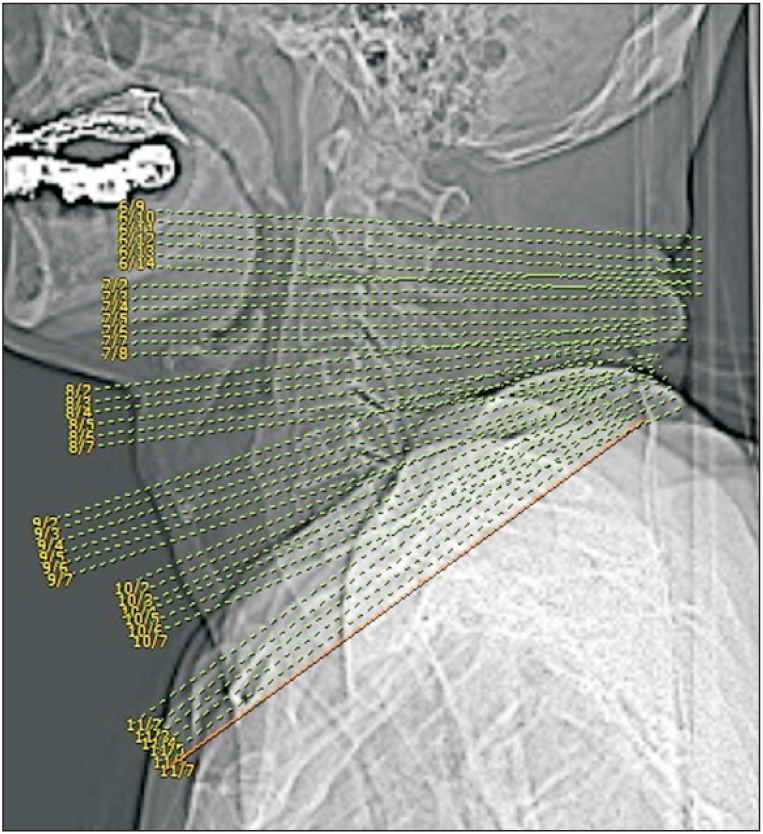
Fig. 2
The anteroposterior diameter (APD) of the spinal canal and the spinal canal area (SCA) were measured in the axial plane at the middle of the vertebral body on the sagittal computed tomography (CT) scan using the polygonal region of interest (ROI) tool of Picture Archiving and Communication System (PACS) preoperatively (A-C) and postoperatively (D-F).
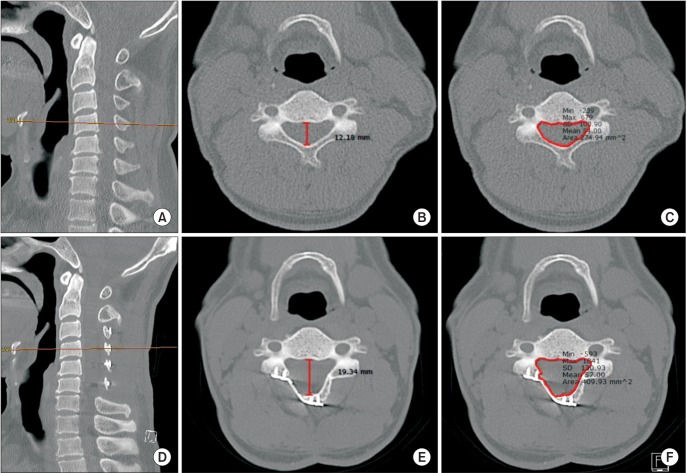
Fig. 3
Measurements of the laminar angle created by one line passing posterior to the transverse foramen and the other line traversing along the inside edge of the lamina on the preoperative (A, B) and postoperative (C, D) computed tomography scans. The difference between the preoperative angle (a) and the postoperative angle (b) was recorded.
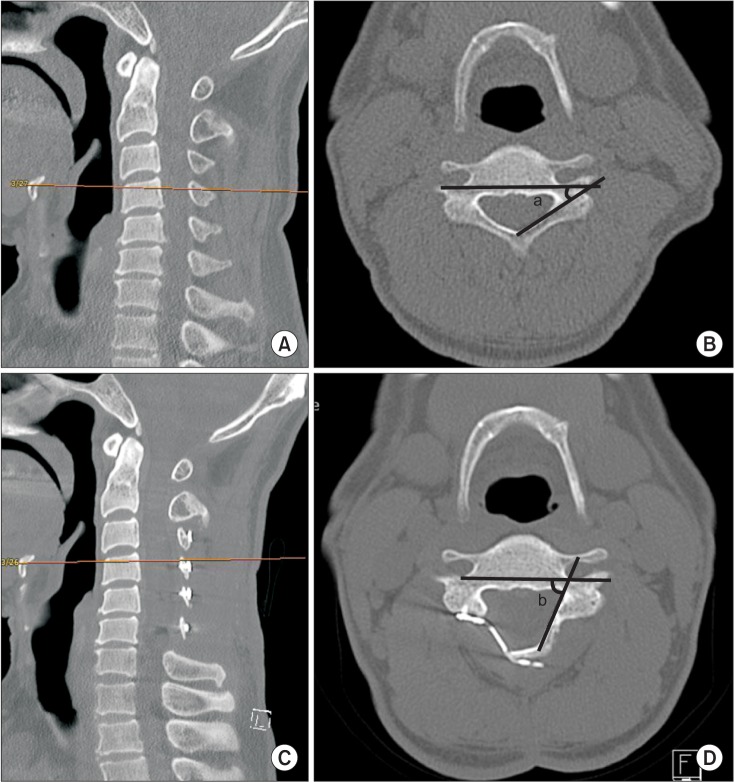
Fig. 4
One way ANOVA analysis of Japanese Orthopedic Association (JOA) recovery rate and ΔLA (changes in laminar angle). The patients were divided into 4 groups based on the ΔLA percentile (group A, < 25%; B, ≥ 25% & < 50%; C, ≥ 50% & < 75%; D, ≥ 75%). Group A showed a significant improvement in JOA score compared to the other groups (*p < 0.05) (N = 49, F = 11.622).
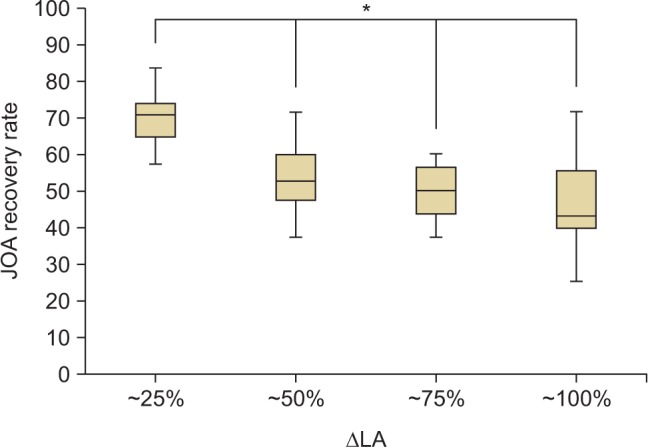
Table 1
Pre- and Postoperative Radiographic Measurements
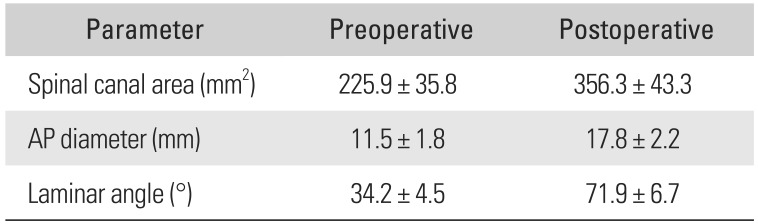
| Parameter | Preoperative | Postoperative |
|---|---|---|
| Spinal canal area (mm2) | 225.9 ± 35.8 | 356.3 ± 43.3 |
| AP diameter (mm) | 11.5 ± 1.8 | 17.8 ± 2.2 |
| Laminar angle (°) | 34.2 ± 4.5 | 71.9 ± 6.7 |
Table 2
Pre- and Postoperative Spinal Canal Area at Each Level
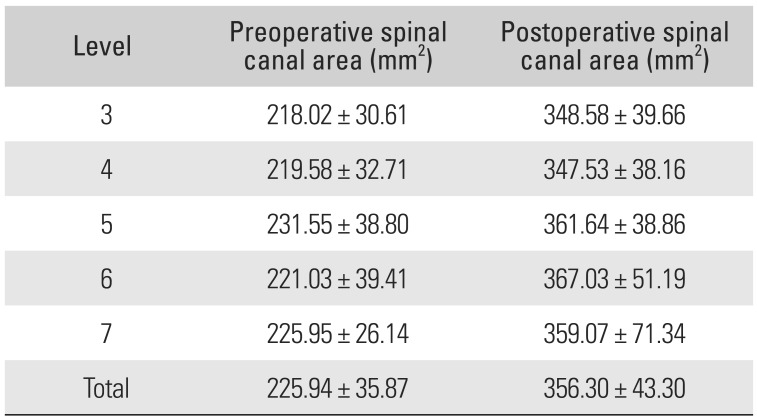
Table 3
Pre- and Postoperative Clinical Evaluation
| Parameter | Preoperative | Postoperative (final follow-up) |
|---|---|---|
| JOA score | 9.1 ± 1.8 (5–13) | 13.4 ± 1.4 (8–16) |
| Visual analog scale | 5.0 ± 1.2 (3–8) | 2.0 ± 0.9 (1–5) |
Table 4
Comparison of Variables from Pearson Correlation Analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download