Abstract
Background
Although both pregabalin and gabapentin are known to be useful for treating lumbar radiating pain and reducing the incidence of surgery, the oral corticosteroids sometimes offer a dramatic effect on severe radiating pain despite the lack of scientific evidence.
Methods
A total of 54 patients were enrolled among 703 patients who complained of lumbar radiating pain. Twenty patients who received an oral corticosteroid was classified as group A and 20 patients who received the control drugs (pregabalin or gabapentin) as group B. Oswestry Disability Index (ODI), Revised Roland Morris disability questionnaire (RMDQ), Short Form 36 (SF-36) questionnaire, lumbar radiating pain, objective patient satisfaction, and objective improvement of patients or physicians were assessed at 2, 6, and 12 weeks after medication.
Results
No difference in the sex ratio and age was observed between the groups (p = 0.70 and p = 0.13, respectively). Group A showed greater improvement in radiating pain after 2, 6, and 12 weeks than group B (p < 0.001, p = 0.001, and p < 0.001, respectively). No differences were observed between the groups in satisfaction at the beginning and 12 weeks after taking the medication (p = 0.062 and p = 0.061, respectively) and in objective improvement of patients and physicians (p = 0.657 and p = 0.748, respectively). Group A was less disabled and had greater physical health scores than group B (p = 0.014 and p = 0.017, respectively).
Lumbar radiating pain is a very common severely painful disorder although it often improves spontaneously without surgery. The recovery rate is approximately 80% within 8 weeks and 95% within 1 year.1) Cohen et al.2) reported that lumbar radiating pain could cause chronic low back pain and substantial economic and social costs.
Although many drugs are used to relieve the symptoms of lumbar radiating pain unless neurological deficit develops, various types of pain relief drugs are often prescribed for patients with lumbar radiating pain.3) Patients diagnosed with sciatica tend to have five times more medications than those diagnosed with low back pain.4)
There are no general recommendations and clear guidelines for prescribing medications for lumbar radiating pain.5) The efficacy and tolerability of pain relief drugs that are usually prescribed for the management of lumbar radiating pain have not been established. The lack of evidence on the efficacy of most lumbar radiating pain managements is at the core of the debate on proper selection of drugs.
Moulin et al.6) reported that both pregabalin and gabapentin are the most commonly prescribed drugs for sciatic radiating pain. Both drugs act on the voltage-gated calcium channel alpha 2-deltal subunit that has a mechanism to relieve neurologic pain.7) They are useful for treating lumbar radiating pain8) and reduce the incidence of surgery.9) In contrast, oral corticosteroids are generally not recommended for back pain accompanied by acute radiating pain or chronic radiating pain because the effects have not been elucidated.10) However, in actual clinical settings, oral corticosteroids sometimes offer a dramatic effect on severe radiating pain despite the lack of scientific evidence.
The purpose of this study was to evaluate whether an oral corticosteroid is not inferior to pregabalin or gabapentin in treating lumbar radiating pain in a randomized controlled trial study.
This study was conducted with full approval of the Daegu Catholic University Medical Center Institutional Review Board (CR-14-049-L). Patient selection process and the overall trial design are shown in Fig. 1.
A total 54 patients were enrolled among 703 patients who complained of lumbar radiating pain or pain needing medication (visual analog scale [VAS] > 3) and who had definite lumbar spinal nerve root compression findings on magnetic resonance imaging (MRI). The period of enrollment was from March 27, 2014 to February 28, 2015. The inclusion and exclusion criteria are presented in Table 1. The patients were sequentially enrolled into the study and prescribed to receive either an oral corticosteroid or control drugs according to the standard protocol: the odd-numbered patients received an oral corticosteroid (group A) and evennumbered patients received the control drugs (group B). Each group was comprised of 20 patients and 7 patients were lost during follow-up (Fig. 1).
Group A patients were prescribed 4 mg triamcinolone (Ledercort; SK Chemical, Seongnam, Korea) twice daily for 2 weeks. Group B patients were prescribed either 7.5 mg pregabalin (Lyrica; Pfizer, Cambridge, MA, USA) twice daily for 2 weeks or 100 mg gabapentin (Neurontin; Pfizer) three times daily for 2 weeks. After the initial prescription, the drugs were tapered or doubled depending on side effects or the therapeutic effect, and the patients were monitored for 12 weeks.
The Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire was completed by all patients at the initial visit to evaluate neurological lumbar radiating pain.11) In addition, the Oswestry Disability Index (ODI) and the Revised Roland Morris disability questionnaire (RMDQ) were completed to evaluate patient functional status. The Short Form 36 (SF-36) questionnaire was used to evaluate the patient's quality of life. Lumbar radiating pain was measured with VAS and objective baseline patient satisfaction was measured with numeric rating scale (NRS) at initial visit. The NRS is an 11-point scale ranging from 0 to 10 with the lower score meaning greater satisfaction. Pain, satisfaction, and objective improvement of patients or physicians were measured at 2, 6, and 12 weeks during the follow-up period. Functional status was evaluated at 6 weeks and 12 weeks, and quality of life was assessed at 12 weeks. The questionnaires were analyzed by a physician who was not involved in this study.
The IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) statistics program for Windows was used for all analyses. Fisher exact test, Student t-test, Wilcoxon signed-rank test, and repeated-measures two factor analysis of variance were used to detected differences between the groups. A p-value < 0.05 was considered statistically significant.
Group A was comprised of 6 males and 14 females, and group B of 7 males and 13 females (Table 2). No difference was detected in the sex ratio between the two groups (p = 0.70). Their mean age when medications were prescribed was 62.5 ± 12.7 years (range, 18 to 78 years): 62.6 ± 13.2 years (range, 18 to 76 years) in group A and 62.4 ± 12.6 years (range, 20 to 78 years) in group B. No difference was observed in age between the groups (p = 0.13).
Radiating pain scores in group A were 4.9 ± 2.9 initially, 3.8 ± 2.8 at 2 weeks after starting the medication, 2.0 ± 2.3 at 6 weeks, and 2.0 ± 2.6 at 12 weeks after starting the medication (Table 3). The scores in group B were 4.8 ± 2.0 initially, 4.3 ± 2.4 after 2 weeks, 3.0 ± 2.2 after 6 weeks, and 3.2 ± 2.2 after 12 weeks of taking the medication. No difference was observed in the scores between the groups at the initial assessment (p = 0.16); however, group A showed greater improvement after 2, 6, and 12 weeks than group B at each time point (p < 0.001, p = 0.001, and p < 0.001, respectively).
The NRS scores for baseline satisfaction of patients in group A were 3.4 ± 0.7 at the beginning of the treatment and 2.6 ± 1.3 after 12 weeks of taking the medication (Table 4). The NRS scores for baseline satisfaction of patients in group B were 3.3 ± 0.7 at the beginning and 2.8 ± 1.1 at 12 weeks after starting the medication. No differences in satisfaction were observed between two groups at the beginning or 12 weeks after taking the medication (p = 0.062 and p = 0.061, respectively). The scores for objective improvement in group A were 2.3 ± 0.9, 2.4 ± 1.0, and 2.3 ± 1.2 at 2, 6, and 12 weeks after taking the medication, respectively. The scores for objective improvement in group B were 2.0 ± 0.7, 2.1 ± 0.9, and 2.0 ± 0.8 at 2, 6, and 12 weeks after taking the medication, respectively. No differences in the magnitude of improvement experienced by the patients were observed between the groups (p = 0.657). The physician's scores of objective improvement of group A were 2.3 ± 1.0, 2.5 ± 1.0, and 2.4 ± 1.1 at 2, 6, and 12 weeks, respectively, after taking the medication. The physician's scores of objective improvement of group B were 2.0 ± 0.7, 2.2 ± 1.0, and 2.0 ± 0.8 at 2, 6, and 12 weeks, respectively, after taking the medication. No differences were observed between the two groups in terms of the objective improvement assessed by the physician (p = 0.748).
Patients in group A were less disabled than those in group B based on the RMDQ scores (p = 0.014) whereas no difference was found in the ODI scores between the groups (p = 0.246) (Table 5).
Group B had higher physical health scores than group A at 12 weeks after taking the medication (p = 0.017), whereas no difference in mental health scores was observed between the groups (p = 0.942).
Sciatica refers to radiating pain down the leg with dermatomal distribution and perhaps additional neurologic deficits.12) It can be caused by mechanical and/or inflammatory reaction that affects lumbosacral nerve roots.13) Sciatica is one of the most common diseases in the outpatient office and observed in approximately 1% of patients with acute low back pain.6141516)
Several conventional treatments have been suggested for lumbar radiating pain, such as pain relief medications (acetaminophen, nonsteroidal anti-inflammatory drugs, muscle relaxants, anti-epileptic drugs [gabapentin and pregabalin], membrane stabilizing agents, and narcotics), spinal manipulation,1) physical therapy, massage, activity as tolerated, and time itself.7891014151718) If patients have intractable pain or progressive neurological deficit, epidural injection or surgical treatment such as decompressive laminotomy or discectomy can be needed.61618) Despite being a mostly self-limiting condition, sciatica is a great loss to society in terms of productivity, treatment costs, and disability even in the long term as well as at the initial episode.151819)
Most studies on the outcomes of lumbar radiating pain have evaluated the results of different surgical and medical treatment methods in patients admitted to the hospital, particularly to the surgical department.12)
Corticosteroids have been reported to decrease swelling in the affected nerve root and reduce sciatic symptoms.20) Haimovic and Beresford21) suggested that corticosteroids may reduce stretching pain evoked by acutely inflamed spinal nerve root in spite of the lack of clinical evidence.17)
Only one blinded, randomized controlled study addressed this issue according to the PuBMed and MEDLINE database.21) In that study, all patients were hospitalized for bed rest for 1 week unlike our study.
Patients in the study of Haimovic and Beresford21) received a 7-day tapering dose of oral dexamethasone. In contrast, patients in our study received a 12-week tapering dose of oral triamcinolone. Haimovic and Beresford21) reported that the efficacy of dexamethasone was no better than placebo in treating sciatic pain unlike our study. There is no consensus regarding the impact of oral glucocorticoid therapy on radiating pain, whereas epidural steroid injection has been associated with short-term improvement.161718222324)
The results of our study conducted in contemporary outpatient setting are different from those of Haimovic and Beresford21) involving hospitalized patients published 20 years ago. Oral steroids did not show excellent efficacy in the study. However, patients in our study who received a corticosteroid such as triamcinolone experienced statistically significant, albeit subtle, improvements. Our results are consistent with those of some previous studies222324) where epidural steroid injection showed greatest benefits at 2–6 weeks after injection. Such effects can be explained by 2 factors: (1) physiological changes that reduce swelling of the affected nerve root through release of pro-inflammatory substances and (2) the cell membrane stabilizing effect of the steroid.20)
Physicians who choose corticosteroids as an initial treatment for sciatica should ensure strict selection of patients with clear-cut signs and symptoms considering that not all patients with back/leg pain have lumbar radiculopathy.19) The physician should contemplate the risks and benefits of corticosteroids on patients, and be aware that potential advantages of this treatment will be effective in the short term based on the current clinical evidence.19)
One of the limitations of our study is the relatively small sample size. In addition, the short follow-up period could have resulted in errors in the incidence of steroid side effects.
In conclusion, oral corticosteroids for the treatment of lumbar radiating pain were more effective in pain relief than gabapentin or pregabalin. The satisfaction of patients and physicians on the drugs and objective improvement status was not inferior to gabapentin or pregabalin. The functional status of oral corticosteroid patients was better than the gabapentin or pregabalin patients based on the RMDQ scores and not inferior to the gabapentin or pregabalin patients according to the ODI scores although the physical health score of pregabalin or gabapentin was superior to that of the oral corticosteroid.
Figures and Tables
 | Fig. 1Flow diagram showing the procedure used in the study. LANSS: Leeds Assessment of Neuropathic Symptoms and Signs, VAS: visual analogue scale. |
Table 1
Inclusion and Exclusion Criteria

Table 2
Characteristics of the Study Sample

| Characteristic | Group A | Group B | p-value |
|---|---|---|---|
| Sex | 0.70 | ||
| Male | 6 | 7 | |
| Female | 14 | 13 | |
| Age, mean ± SD | 62.5 ± 12.7 | 62.6 ± 13.2 | 0.13 |
Table 3
Comparison of Radiating Pain
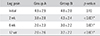
| Leg pain | Group A | Group B | p-value |
|---|---|---|---|
| Initial | 4.9 ± 2.9 | 4.8 ± 2.0 | 0.16 |
| 2 wk | 3.8 ± 2.8 | 4.3 ± 2.4 | < 0.001* |
| 6 wk | 2.0 ± 2.3 | 3.0 ± 2.2 | 0.001* |
| 12 wk | 2.0 ± 2.6 | 3.2 ± 2.2 | < 0.001* |
Table 4
Comparison of Treatment Satisfaction
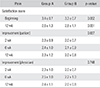
Table 5
Comparison of Disability and Quality of Life

ACKNOWLEDGEMENTS
This work was supported by a grant from the Research Institute of Medical Science, Catholic University of Daegu in 2014.
References
1. Legrand E, Bouvard B, Audran M, Fournier D, Valat JP. Spine Section of the French Society for Rheumatology. Sciatica from disk herniation: medical treatment or surgery? Joint Bone Spine. 2007; 74(6):530–535.

2. Cohen SP, Wenzell D, Hurley RW, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007; 107(1):99–105.

3. Vogt MT, Kwoh CK, Cope DK, Osial TA, Culyba M, Starz TW. Analgesic usage for low back pain: impact on health care costs and service use. Spine (Phila Pa 1976). 2005; 30(9):1075–1081.

4. Selim AJ, Ren XS, Fincke G, et al. The importance of radiating leg pain in assessing health outcomes among patients with low back pain: results from the Veterans Health Study. Spine (Phila Pa 1976). 1998; 23(4):470–474.
5. Koes BW, van Tulder M, Lin CW, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010; 19(12):2075–2094.
6. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007; 12(1):13–21.
7. Field MJ, Li Z, Schwarz JB. Ca2+ channel alpha2-delta ligands for the treatment of neuropathic pain. J Med Chem. 2007; 50(11):2569–2575.
8. Saldana MT, Navarro A, Perez C, Masramon X, Rejas J. Patient-reported-outcomes in subjects with painful lumbar or cervical radiculopathy treated with pregabalin: evidence from medical practice in primary care settings. Rheumatol Int. 2010; 30(8):1005–1015.

9. Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg. 2010; 110(4):1180–1185.

10. Bigos SJ, Bowyer RO, Braen GR, et al. Acute lower back problems in adults: clinical practice guideline No. 14. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services;1994.
11. Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92(1-2):147–157.

12. Balague F, Nordin M, Sheikhzadeh A, et al. Recovery of severe sciatica. Spine (Phila Pa 1976). 1999; 24(23):2516–2524.

13. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002; 5(2):182–199.

14. Ogawa S, Satoh J, Arakawa A, Yoshiyama T, Suzuki M. Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west. Drug Saf. 2012; 35(10):793–806.

16. Scheer SJ, Radack KL, O'Brien DR Jr. Randomized controlled trials in industrial low back pain relating to return to work. Part 2: discogenic low back pain. Arch Phys Med Rehabil. 1996; 77(11):1189–1197.

17. Deyo RA. Back pain revisited: newer thinking on diagnosis and therapy. Consultant. 1993; 33(2):88–96.
18. Weber H. The natural history of disc herniation and the influence of intervention. Spine (Phila Pa 1976). 1994; 19(19):2234–2238.

19. Holve RL, Barkan H. Oral steroids in initial treatment of acute sciatica. J Am Board Fam Med. 2008; 21(5):469–474.

20. Starkweather A, Witek-Janusek L, Mathews HL. Neural-immune interactions: implications for pain management in patients with low-back pain and sciatica. Biol Res Nurs. 2005; 6(3):196–206.

21. Haimovic IC, Beresford HR. Dexamethasone is not superior to placebo for treating lumbosacral radicular pain. Neurology. 1986; 36(12):1593–1594.

22. Ridley MG, Kingsley GH, Gibson T, Grahame R. Outpatient lumbar epidural corticosteroid injection in the management of sciatica. Br J Rheumatol. 1988; 27(4):295–299.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download