Abstract
Background
As surgical complications tend to occur more frequently in the beginning stages of a surgeon's career, knowledge of perioperative complications is important to perform a safe procedure, especially if the surgeon is a novice. We sought to identify and describe perioperative complications and their management in connection with minimally invasive transforaminal lumbar interbody fusion (TLIF).
Methods
We performed a retrospective chart review of our first 124 patients who underwent minimally invasive TLIF. The primary outcome measure was adverse events during the perioperative period, including neurovascular injury, implant-related complications, and wound infection. Pseudarthroses and adjacent segment pathologies were not included in this review. Adverse events that were not specifically related to spinal surgery and did not affect recovery were also excluded.
Results
Perioperative complications occurred in 9% of patients (11/124); including three cases of temporary postoperative neuralgia, two deep wound infections, two pedicle screw misplacements, two cage migrations, one dural tear, and one grafted bone extrusion. No neurologic deficits were reported. Eight complications occurred in the first one-third of the series and only 3 complications occurred in the last two-thirds of the series. Additional surgeries were performed in 6% of patients (7/124); including four reoperations (two for cage migrations, one for a misplaced screw, and one for an extruded graft bone fragment) and three hardware removals (one for a misplaced screw and two for infected cages).
Conclusions
We found perioperative complications occurred more often in the early period of a surgeon's experience with minimally invasive TLIF. Implant-related complications were common and successfully managed by additional surgeries in this series. We suggest greater caution should be exercised to avoid the potential complications, especially when surgeon is a novice to this procedure.
Minimally invasive transforaminal lumbar interbody fusion (TLIF) has shown the benefits over conventional lumbar fusion in terms of the amount of intraoperative blood loss, the intensity of postoperative pain, and the hospital stay duration.1,2,3,4,5) As with other surgical procedures, however, minimally invasive TLIF also showed complications related to the surgeon's learning curve.6,7) Although complication rates have varied, most literature reports a common set of complications, including neurologic deficits, dural tear, wound infection, screw misplacement, and cage migration.1,2,3,4,5,6,7)
Because surgical complications may occur more often in the early period of surgeon's experience with the procedure, knowledge of perioperative complications is important in order to perform a safe procedure, especially if the surgeon is a novice. We therefore sought to identify and describe perioperative complications and their management associated with minimally invasive TLIF.
We reviewed clinical and radiographic data for the first 124 patients undergoing minimally invasive TLIF. After approval by National Health Insurance Service Ilsan Hospital Institutional Review Board, we queried our institution's database to identify patients who underwent this procedure at least 5 years earlier (from October 2003 to May 2007). Two independent investigators reviewed the data for the patient's demographics, procedures, disposition, and perioperative and postoperative complications. Data on adverse events during the perioperative period (day of surgery to 12 weeks postoperatively) were included in this study.
We performed all of our surgical procedures for minimally invasive TLIF as follows. Under fluoroscopic guidance, a 22-mm diameter METRx tubular retractor (Medtronic, Memphis, TN, USA) was introduced through a 2.5-cm incision for both neural decompression and to access the interbody space in all patients. The approach was carried out on the side with the worst preoperative radiculopathy. Sextant screws and rods (Medtronic) were placed percutaneously on the contralateral side to distract the interbody space and maintain the distracted position. Once the optimal interbody distraction has been achieved, endplate preparation was performed through the tubular retractor using curettes and endplate scrapers. After all of the cartilaginous endplate was removed, the autogenous bone obtained from the resected lamina and facet was mixed with demineralized bone matrix (Osteofil RT DBM paste, Regeneration Technologies Inc., Alachua, FL, USA) and placed anteriorly and contralateral to the annulotomy within the interbody space in all cases. A polyetheretherketone (Capstone, Medtronic) was then inserted into the disc space. Ipsilateral percutaneous pedicle screws and rods were then placed through the same incision. No additional contralateral facet fusion was performed.
Reported complications included dural tears, screw misplacement, cage migration, postoperative transient neuralgia, neurologic deficits, deep wound infections, and instrumentation failures. Pseudarthroses and adjacent segment disease were not included in this review. Adverse events that were not specifically related to the spinal surgery and did not affect recovery (for example, urinary tract infections, ileus, and anemia) were also excluded. Postoperative transient neuralgia was identified as a pain that patients experienced postoperatively in a short period of time not lasting more than 3 months. The pain radiated down to the patient's leg regardless of its cause. Postoperative motor deficits were identified using a manual muscle strength test, with a deficit defined as a score ≤ 4 on a scale of 0 to 5. Deep wound infection was defined as a deep infection requiring a second surgical procedure, such as debridement and implant removal. Repeat surgical procedures during the perioperative period were documented as revisions, removals, supplemental fixations, or reoperations, depending on the clinical circumstances.
The mean age of patients at the time of surgery was 59.3 years (range, 23 to 82 years). There were 45 male (36%) and 79 female (64%) patients. The mean body mass index was 25.0 ± 3.5 kg/m2 (range, 18.2 to 42.7 kg/m2). Thirty patients (24%) were current smokers at the time of surgery. There were 26 diabetic patients (21%) and 29 (T-score < -2.5) patients (23%) with osteoporosis. Sixty-seven patients (54%) reported at least one coexisting medical disease (range, 1 to 5 conditions). Eight patients had previously undergone back surgery (5 microdiscectomy and 3 laminectomy) prior to the current surgery.
One hundred forty-one intervertebral segments were treated with minimally invasive TLIF. The most commonly treated segment was L4-5 (61%), followed by L5-S1 (21%), L5-6 (10%), and L3-4 (8%). One hundred eight patients (87%) were treated with a single-level fusion surgery. There were 15 cases (12%) of double-level arthrodesis, and one case that required a 3-level fusion surgery. Preoperative diagnosis of each treated segment included 75 segments (53%) with spondylolisthesis (40 segments with degenerative spondylolisthesis, 35 segments with spondylolytic spondylolisthesis), 44 segments (31%) with foraminal stenosis and/or foraminal disc herniation, 11 segments (8%) with degenerative segmental instability, five segments with recurrent disc herniation, three segments with postlaminectomy instability, and three segments with degenerative disc disease.
The mean operative time was 176.3 ± 38.7 minutes (range, 80 to 305 minutes) for a single-level surgery and 235.0 ± 27.9 minutes (range, 180 to 270 minutes) for a multi-level surgery. The average single-level intraoperative blood loss was 238.4 ± 183.0 mL (range, 50 to 800 mL) and 329.4 ± 256.4 mL (range, 50 to 900 mL) for multi-level surgery. Blood transfusion was identified in 16% of single-level surgery patients (17/108) and 38% of the multi-level surgery patients (6/16). The mean hospital stay was 7.5 ± 5.4 days (range, 1 to 37 days) in single-level surgery and 10.3 ± 10.8 days (range, 2 to 45 days) in multi-level surgery.
Perioperative complications occurred in 9% of patients (11/124); including 3 cases of temporary postoperative neuralgia, two deep wound infections, two pedicle screw misplacements, two cage migrations, one dural tear, and one grafted bone extrusion. No neurologic deficits were reported. Furthermore, most of perioperative complications occurred during the earlier portion of the series rather than the latter portion (eight vs. three events). Eight complications occurred in the first one-third of the series (patients #5, #11, #13, #18, #27, #36, #39, and #45) and only 3 complications occurred in the last two-thirds of the series (patients #70, #117, and #121) (Fig. 1).
Additional surgeries were performed in 6% of patients (7/124) in the perioperative period. Four reoperations (two for cage migrations, one for a misplaced screw, and one for an extruded graft bone fragments) and three hardware removals (one for a misplaced screw and two for infected cages) were performed in the perioperative period.
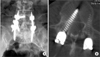
Two symptomatic pedicle screw misplacements (patients #5 and #45) were noted postoperatively at the caudal pedicle wall of L4 and the medial wall of L4, respectively. In patient #5, a 66-year-old woman with spondylolytic spondylolisthesis at L4, the caudally misplaced screw was removed without a replacement screw, leaving a unilateral fixation. Her radicular leg pain from the misplaced screw resolved immediately after the second surgery and she had a solid fusion two years postoperatively. In patient #45, a 56-year-old woman with double-level spondylolytic spondylolisthesis in L4 and L5, the misplaced screw was removed and replaced in the correct position. Immediate following the surgery, her symptoms from the misplaced screw disappeared; despite this, a pseudarthrosis was identified at her two- and five-year follow-up visits (Fig. 2).
Patient #27, an 82-year-old man with foraminal stenosis at L5-1, and patient #36, a 63-year-old woman with degenerative spondylolisthesis at L5, both experienced postoperative radicular leg pain due to cage migration (Fig. 3). Immediate symptom relief was achieved after repeated surgical intervention to replace the migrated posterior cage in the correct position. Patient #11, a 68-year-old woman with postlaminectomy instability at L4-5, underwent a second operation to remove bony fragments extruding from previously grafted bone material in the intervertebral space that was irritating the traversing nerve root and presented as postoperative lumbar radiculopathy. After successful removal of the extruding bony fragments, her symptoms resolved completely (Fig. 4).
Patient #117, a 57-year-old man with recurrent disc herniation at L4-5, experienced a small sized dural tear during the decompression procedure. The torn dura was repaired through the enlarged surgical corridor with an expandable retractor after extension of the skin incision from the original surgical wound. Subsequently, there was no evidence of cerebrospinal fluid (CSF) leak or pseudomeningocele during the follow-up period.
Patients #13 and #70 had courses complicated by deep wound infections. Patient #13, a 61-year-old man who underwent two-level surgery for degenerative disc disease at L4-5 and segmental instability at L5-6, experienced worsening back pain and generalized fever with concurrent elevation of his C-reactive protein level during the two months following his initial surgery. The magnetic resonance imaging (MRI) obtained at two months postoperatively showed an enhancing area around the intervertebral space with a cage subsidence, indicating a deep wound infection at the level of L4-5 without significant involvement of the previous posterior surgical wound. The anterior retroperitoneal approach was utilized to remove the infected cage and for surgical debridement two months after the initial surgery. At the same time, an anterior interbody fusion was performed using a tricortical strut graft harvested from the left iliac crest. After the second surgery, parenteral antibiotics were continued until the elevated C-reactive protein level was normalized. Eventually, the infection was controlled and a solid fusion was documented at his two-year follow-up.
Patient #70, a 58-year-old man with recurrent disc herniation at L4-5, suffered from increasing back pain and a sustained fever postoperatively as well as elevated C-reactive protein levels. His postoperative MRI showed peripherally rim-enhancing abscess formation at the approach side of the initial surgery. The patient then underwent concurrent interbody fusion using tricortical strut grafts harvested from the posterior iliac crest and infected cage removal with surgical debridement through an extension of the previous posterior wound with an expandable retractor. After the second surgery, parenteral antibiotics were maintained until the elevated C-reactive protein level was normalized. Fortunately, his infection was ultimately controlled and the grafted bone at the infected intervertebral segment healed successfully.
Minimally invasive TLIF is an innovative technique that has recently developed with a growing interest in the field of less invasive surgical fusion techniques.8) Mastering the minimally invasive procedures can be quite different from learning the traditional procedures. In our study, the overall occurrence rate of perioperative complications was 9% and 72% of the complications occurred in the first 1/3 of the series. Our result suggests that the minimally invasive TLIF procedure is challenging, especially when one is beginning to learn it.
The lack of clear anatomic landmarks appears to be a significant limitation in the minimally invasive approach to the spine. Even if the procedure performed under proper fluoroscopic guidance, the misplacement of pedicle screws is possible; the rate of malpositioned screws ranges between 0.35% and 13%.9) Computed tomography scans could be useful tools for identifying abnormal pedicle screw placement. We experienced two cases (1.6%) that required an additional surgery to reposition a malpositioned screw. We offer five surgical tips to avoid pedicle screw misplacement: (1) depending on the patient's size, it may be necessary to modify the skin incision site by obtaining the proper distance lateral from the midline, measured from the preoperative axial images of an MRI; (2) obtain a true anteroposterior fluoroscopic view of the target vertebrae to get a clear radiographic image; (3) have sufficient time to get an appropriate medial to lateral trajectory of the guide needle until its tip appears at the lateral cortical margin of pedicle in the anteroposterior fluoroscopic view; (4) spend a time necessary to obtain a tactile feeling with the needle tip and try to palpate the bony junction between lateral facet wall and transverse process to select an optimal entry point; and (5) avoid driving screws too much deeper than necessary to prevent the screw tulip from resting on the facet joint.
Cage migration is also known to be a serious complication. The migration can be classified as posterior, anterior, or sagittal. Posteriorly migrated cages could compress the nerve root or dura and might intensify neurological symptoms. All of our cases of migration were posterior and needed an additional surgery to resolve the symptoms. The number, shape, size and implantation site of the cages could influence cage migration. Aoki et al.10) reported that the use of bullet-shaped cages, higher posterior disc height, the presence of scoliotic curvature, and undersized fusion cages were possible risk factors for cage migration.
Potential occult injury to exiting nerve root or dura could be considered as a downside of minimally invasive TLIF but it is unclear. In our cases, there was no occult nerve root injury. However, we demonstrated three cases resulting in transient postoperative neuralgia and one case of a dural tear. We do not know the exact cause of the postoperative neuralgia, but could presume that it might be related to the surgical procedure or is a symptom related to the healing process of damaged neural tissue after decompression. To clarify this issue, further investigations should be undertaken. A dural tear with CSF leakage can prevent healing, leading to wound breakdown, infection, and other clinical sequelae. In our series, there was one case of a dural tear and the tear was successfully repaired without any neurologic sequelae. Repairing a dural tear within the limited operating field may be quite challenging, and could require an additional extension of the skin incision. The present study has a number of limitations. The design of the study is retrospective in nature and the patients were not randomly selected. In addition, the cohort size of this study is not large enough to have sufficient statistical power for drawing complete conclusions. As the current study was based on the data from a single surgeon's experience, the data could not be generalized. Furthermore, there may be a selection bias in that the surgeon may preferentially choose straightforward cases early in his or her experience with a new minimally invasive technique.
Despite these weaknesses, our data shows that the minimally invasive TLIF procedure demonstrates higher complications, especially in the early stage of surgeon's learning curve. The procedure is challenging initially. The results of our study correspond to previous reports finding that the overall complications of minimally invasive TLIF occur within the initial 30 cases of a surgeon's attempts at the procedure.1,4,6) While our study provides some value for clinical reference, future studies from a larger randomized controlled trial is warranted.
Our data suggest that perioperative complications of minimally invasive TLIF occurred more often in the early period of a surgeon's experience with the procedure. Cage- or pedicle screw-related complications were the most common and were managed by additional surgeries in this series. Furthermore, greater caution should be exercised to avoid the potential complications until surgeon's has performed at least 45 procedures.
Figures and Tables
Fig. 1
Eight complications occurred in the first one-third of the series (patients #5, #11, #13, #18, #27, #36, #39, and #45) and only 3 complications occurred in the last two-thirds of the series (patients #70, #117, and #121).

Fig. 2
A medially misplaced screw in the right side L4 pedicle demonstrated symptomatic L4 radiculopathy (arrow) in the postoperative radiography (A) and axial view of computed tomography scan (B).
References
1. Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008; 9(6):560–565.

2. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976). 2009; 34(13):1385–1389.

3. Scheufler KM, Dohmen H, Vougioukas VI. Percutaneous transforaminal lumbar interbody fusion for the treatment of degenerative lumbar instability. Neurosurgery. 2007; 60:4 Suppl 2. 203–212.

4. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop. 2009; 33(6):1683–1688.

5. Shunwu F, Xing Z, Fengdong Z, Xiangqian F. Minimally invasive transforaminal lumbar interbody fusion for the treatment of degenerative lumbar diseases. Spine (Phila Pa 1976). 2010; 35(17):1615–1620.

6. Lau D, Lee JG, Han SJ, Lu DC, Chou D. Complications and perioperative factors associated with learning the technique of minimally invasive transforaminal lumbar interbody fusion (TLIF). J Clin Neurosci. 2011; 18(5):624–627.

7. Lee JC, Jang HD, Shin BJ. Learning curve and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion: our experience in 86 consecutive cases. Spine (Phila Pa 1976). 2012; 37(18):1548–1557.

8. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Minimally invasive or open transforaminal lumbar interbody fusion as revision surgery for patients previously treated by open discectomy and decompression of the lumbar spine. Eur Spine J. 2011; 20(4):623–628.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download