Abstract
Background
Metastatic pathological fractures of the spine are a major problem for cancer patients; however, there is no consensus on treatment strategy. The purpose of this study was to evaluate various treatment options by analyzing their patterns for metastatic pathological fractures of the spine.
Methods
In this study, 54 patients (male:female = 36:18) who were diagnosed with metastatic pathological fractures of spine were recruited. Demographic data, origin of cancer, type of treatment, and results were obtained from electronic medical records. Treatment options were divided into radiotherapy (RT), vertebroplasty (VP) or kyphoplasty (KP), operation (OP), and other treatments. Treatment results were defined as aggravation, no response, fair response, good response, and unknown. The survival time after detection of pathologic fractures was analyzed with the Kaplan-Meier method.
Results
The mean age of the patients was 62.3 years. Hepatocellular carcinoma was the most common cancer of primary origin (n = 9), followed by multiple myeloma (n = 8). RT was the most common primary choice of treatment (n = 29, 53.7%), followed by OP (n = 13, 24.1%), and VP or KP (n = 10, 18.5%). Only 13 of 29 RT cases and 7 of 13 OP cases demonstrated a fair or good response. The mean survival time following detection of pathological spinal fractures was 11.1 months for 29 patients, who died during the study period.
Conclusions
RT was the most common primary choice of treatment for metastatic pathological fractures of the spine. However, the response rate was suboptimal. Although OP should be considered for the relief of mechanical back pain or neurologic symptoms, care should be taken in determining the surgical indication. VP or KP could be considered for short-term control of localized pain, although the number of cases was too small to confirm the conclusion. It is difficult to determine the superiority of the treatment modalities, hence, a common guideline for the diagnosis and treatment of metastatic pathological fractures of the spine is required.
Spinal metastasis is the most frequent bone metastasis. It can result in axial or radicular pain or motor weakness by tumor invasion of the neural structure. Pathological spinal fractures usually cause severe pain or progressive neurological deficits, hence, prevention of pathological fractures is a primary goal in the management of spinal metastasis.1) However, diagnosis of spinal metastasis is frequently delayed, since the onset of symptoms occurs following neural tumor invasion or the development of pathological fractures in many cases. Therefore, metastatic pathological fractures of the spine are the initial manifestation in many cases of spinal metastasis.
The management of metastatic spinal tumors, including cord compression and pain control has been the focus of numerous studies.23) Plausible treatment options for metastatic spinal tumor patients include chemotherapy, steroid injections, radiotherapy (RT), vertebroplasty (VP)/kyphoplasty (KP), or operation (OP).4567) The indications and limitations of the various forms of treatment have been addressed in various studies. However, until now, there are very few studies on the management of pathological spinal fractures by metastasis alone, although these may be of importance as clinical manifestations are often identified.
The aim of this study was to evaluate the role and efficacy of various treatment options by reviewing the medical records of patients diagnosed with pathological spinal fractures due to metastasis. This is of importance, as spinal metastasis is frequently detected simultaneous with the diagnosis of pathological fractures. In addition, we aimed to establish the basis for specific guidelines for the management of pathological spinal fractures by metastasis.
A retrospective case record review study was performed using the institutional program Asan Biomedical Research Environment. This program is used to search for patients and their electronic medical records (EMR) while guaranteeing anonymity. A search was conducted for the period between January 2008 and December 2012, by entering the search terms 'metastatic tumor of the spine' and 'pathological fracture of the spine' in the program. Fifty-four of the 77 patients that were found on using the search terms were included in this study. Reasons for exclusion of the 23 cases were benign compression fractures (n = 11), pathological fractures due to infection (n = 5), and insufficient information (n = 7).
Demographic data including sex, age, primary cancer, and presence of metastasis to other organs were collected by reviewing the EMR of the patients. Information concerning spinal pathological fractures included related symptoms, initial oncologist consultation at the department, primary treatment methods, and secondary treatment methods. The date of diagnosis for primary cancer, spinal metastasis, pathological fractures, and confirmed death were also collected. The characteristics of spinal metastasis, including the number of metastatic spinal tumors or location of pathological fractures, were obtained by reviewing the picture archiving communication system. The Institutional Review Board of our institution approved the study and the requirement for informed consent was waived due to the retrospective nature of the study.
Treatment options for pathological spinal fractures were divided as follows: (1) observation; (2) RT; (3) VP/KP; (4) OP; and (5) other treatments. Other treatment options included steroid therapy or bracing. OP was considered in case of intractable pain or progressive neurologic deficit. RT or VP/KP was the preferred option if the main symptom was pain without neurologic deficit. Observation or bracing were considered if the symptom was minimal.
The treatment results were defined as follows: (1) aggravation; (2) no response; (3) fair response; (4) good response; and (5) unknown. Each category was assigned on reviewing changes in subjective symptoms between preand post-treatment periods. A fair response was defined as a slight decrease in pain but persistent residual pain, or the disappearance of pain following each treatment, which recurred within 3 months. A good response was defined as the disappearance of pain for > 3 months following each treatment. Treatment results from each patient's EMR were independently reviewed by two raters. Any discrepancy in decision was resolved by mutual consensus between the two raters.
Demographic data, distribution of primary cancer, sites of pathological fractures, number of metastatic spinal tumors and other organ metastasis, symptoms, first treatment option, second treatment option, and treatment results were analyzed descriptively. The survival time after detection of pathological spinal fractures was analyzed by the Kaplan-Meier method. Factors influencing survival time were analyzed by the log-rank test. Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant.
Of the 54 patients included in the study, 36 were male (66.7%) and 18 were female (33.3%). The mean age of the patients was 62.3 ± 12.5 years. The overall primary origin of cancer in the patients was summarized in Table 1. The most common origin of cancer was hepatocellular carcinoma (n = 9), followed by multiple myeloma (n = 8), lung cancer (n = 7), and breast cancer (n = 7). The numbers of organ metastases excluding the spine were as follows: no other organ metastasis (11/54, 20.4%), metastasis of 1 additional organ (22/54, 40.7%), and metastasis of ≥ 2 organs (21/54, 38.9%). Pain including back pain or radiating pain was the main symptom found in 40 patients, 13 patients demonstrated pain with motor weakness and no symptoms were found in the remaining patient.
The sites of the pathological spinal fractures were demonstrated in Fig. 1. The most commonly involved fracture sites were T11, L1, L3, and L4. The number of spinal metastases was as follows: 1 (14/54, 25.9%), 2 (5/54, 9.3%), 3 (4/54, 7.4%), and ≥ 4 (31/54, 57.4%).
Primary treatment options to control pathological spinal fractures were briefly illustrated in Fig. 2. RT was the most commonly used form of treatment (29/54, 53.7%). The results of primary treatment were summarized in Table 2. No response or aggravation was found in 40.8% of patients. The results of each treatment option were shown in Table 3. Negative results were found in 44.8% (13/29) of patients who underwent RT. Patients who underwent OP procedures showed variable results. A fair response was observed in 50% of patients (5/10) who underwent VP or KP.
In cases where primary treatment failed, secondary treatment was attempted. Eight patients who did not respond to RT were recommended to undergo VP or KP (4 cases) and OP (4 cases). Fig. 3 schematically shows the stages of treatment flow.
The mean period between detection of primary cancer and spinal metastasis was 10.8 ± 18.8 months (range, 0 to 79 months). The mean period between detection of spinal metastasis and detection of pathological spinal fractures was 0.9 ± 2.5 months (range, 0 to 12 months). The mean follow-up period following detection of pathological spinal fractures was 14.4 ± 18.6 months. During the follow-up period, 29 patients were confirmed as dead. In the 29 deceased patients, the mean survival time following detection of pathological spinal fractures was 11.1 ± 8.8 months. The Kaplan-Meier survival curve was shown in Fig. 4. The expected median survival was 20.0 months (95% confidence interval, 9.7 to 30.3 months).
The differences in survival time after detection of pathological spinal fractures in relation to the origin of cancer were shown in Table 4. Survival time in hepatocellular carcinoma was significantly shorter than in multiple myeloma (p = 0.027) (Fig. 5). Survival time in relation to age was not different between groups (p = 0.218).
Pathological metastatic fractures of the spine may lead to a debilitated state due to severe pain and possible motor weakness. The results of the present study indicated that most pathological fractures were detected simultaneous to the diagnosis of spinal metastasis. This finding suggested that spinal metastasis is primarily asymptomatic, and therefore, difficult to detect during the early stage.
Treatment should aim to relieve the mechanical instability and neural compression that primarily lead to symptoms of pathological spinal fractures. Thus, surgical decompression and stabilization with instrumentation would be an ideal treatment option. Many studies have suggested good clinical outcomes following surgical treatment including pain control, and regaining or maintaining mobility.891011) In addition, quality of life reportedly improves following palliative surgery.8) Furthermore, palliative surgery is considered a valuable treatment option for patients and their families.12) However, surgical treatment does not always guarantee a good outcome according to several studies. A previous study showed that palliative surgery is of benefit to only half of the patients.10) In addition, a higher morbidity rate following emergency surgery for spinal metastasis is suggested.13) Attempts at minimally invasive techniques to reduce postoperative complications lead to a good outcome.1415) In this study, approximately 50% of patients who underwent open surgery demonstrated fair or good outcomes, corroborating previous reports. Patients who had lost the ability to walk or presented with debilitating preoperative general health did not respond well to surgical treatment. Therefore, decision making in surgical indication is considered of critical importance. Various factors such as primary cancer type, preoperative ambulation, or performance status should be considered when deciding on surgical treatment.716)
In this study, RT was the most common initial treatment used by oncologists. The advantages of RT include its relative easy use, effective pain control, and avoidance of systemic complications that may occur in chemotherapy. However, RT may not be suitable for control of pathological spinal fractures, as it theoretically may not restore mechanical stability.
Studies on the efficacy of RT for pathological spinal fractures by metastasis are rare. Numerous studies have suggested the efficacy of RT against a broad spectrum of spinal metastases, with or without pathological fractures, and also for pain control, regardless of the pathological fractures.1718) Stability is reportedly maintained following RT in osteolytic metastasis of breast cancer, nevertheless, this finding has not been supported in any other studies on accompanying pathological spinal fractures.19) Pain control was not improved in many patients that underwent RT as a primary treatment option, hence RT should be selected with caution in patients with pathological spinal fractures due to metastasis. In addition, progression of neurological symptoms may occur by delaying the onset of surgical treatment with the initial option of RT. Recently, stereotactic radiosurgery (SRS) has been suggested as an alternative to conventional external body RT.2021) However, we did not consider SRS in our study, as it has not been widely applied in our hospital.
VP or KP is another useful option for metastatic pathological fractures of the spine. A number of studies have reported on the efficacy of VP or KP in controlling pathological spinal fractures.462223) The results of our study and previous studies collectively indicate that VP or KP may be used to control mechanical back pain, at least for a short period of time.
Pathological spinal fractures may be an indication of potential poor outcomes. In our study, the expected survival time after the onset of pathological spinal fractures was < 2 years. In addition, the mean survival time of deceased patients was approximately 1 year. However, survival time may vary with the origin of cancer. Although we observed different survival times between patients with hepatocellular carcinoma and multiple myeloma, this might be a result of the small sample size. The finding of no difference in survival time based on age may be due to the mere presence of the pathological spinal fracture implying the terminal stage of the disease.
There were several limitations in the current study. First, because the study population was selected based on specific search terms, many cases were eliminated in the process. The exact diagnostic term 'pathologic spinal fractures' was not documented in a number of cases. Second, the regimen of RT was not collective, and OP or VP/KP was performed by different surgeons. This might have led to biased results. Third, specific tools for outcome measurements were not applicable due to the retrospective nature of the study that involved many departments such as oncology, neurosurgery, therapeutic radiology, and orthopedic surgery. For this reason, we utilized subjective patient symptoms as outcome measurements based on chart reviews.
Regardless of these limitations, our study provides information on the management of metastatic pathological fractures of the spine. In addition, this study suggests plausible indications and limitations of the treatment modalities for the treatment of metastatic pathological fractures of the spine.
RT is a widely used procedure for metastatic pathological fractures of the spine despite its suboptimal clinical outcome. RT could be considered for pain control in mechanically stable and neurologically intact cases. However, close observation is mandatory because of high failure rate in case of pathologic fractures. OP may be considered to selectively control mechanical back pain and relieve neurologic compromise. However, thorough review of general health status, neurologic status, expected survival, origin of cancer, and underlying diseases of patients is required because negative outcomes are frequently found. VP or KP is a useful treatment option for short-term control of local pain, although the number of cases was too small to confirm the conclusion. However, a common guideline in the diagnosis and treatment of metastatic pathological spinal fractures is necessary due to the difficulty faced in determining the most optimal and superior treatment modality. Further studies with a larger sample size are required to elucidate the role of each treatment modality in the control of metastatic pathological fractures of the spine.
Figures and Tables
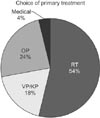 | Fig. 2Distribution of primary treatment options for pathological spinal fractures by metastasis. RT: radiotherapy, VP: vertebroplasty, KP: kyphoplasty, OP: operation. |
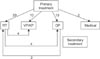 | Fig. 3The stages of treatment flow. RT: radiotherapy, VP: vertebroplasty, KP: kyphoplasty, OP: operation. |
 | Fig. 5Difference in survival time between hepatocellular carcinoma and multiple myeloma (p = 0.027 by log-rank test). |
Table 1
Primary Origins of Metastatic Spinal Tumors

Table 2
Clinical Results of First Line Treatment for Metastatic Pathological Fractures of the Spine

| Response | No. of cases (%) |
|---|---|
| Aggravation | 9 (16.7) |
| No change | 13 (24.1) |
| Fair response | 19 (35.2) |
| Good response | 8 (14.8) |
| Unknown | 5 (9.2) |
| Total | 54 (100.0) |
Table 3
Cross Table Showing the Response by Each Treatment Modality

| Treatment | Response | |||||
|---|---|---|---|---|---|---|
| Aggravation | No change | Fair | Good | Unknown | Total | |
| Radiotherapy | 7 | 6 | 11 | 2 | 3 | 29 |
| Vertebroplasty/kyphoplasty | 0 | 3 | 5 | 1 | 1 | 10 |
| Operation | 2 | 3 | 3 | 4 | 1 | 13 |
| Medical | 0 | 1 | 0 | 1 | 0 | 2 |
| Total | 9 | 13 | 19 | 8 | 5 | 54 |
Table 4
Differences of Survival Time by Origins of Cancers after Detection of Metastatic Pathological Fractures of the Spine

References
2. Savage P, Sharkey R, Kua T, et al. Malignant spinal cord compression: NICE guidance, improvements and challenges. QJM. 2014; 107(4):277–282.

3. L'esperance S, Vincent F, Gaudreault M, et al. Treatment of metastatic spinal cord compression: cepo review and clinical recommendations. Curr Oncol. 2012; 19(6):e478–e490.
4. Ha KY, Min CK, Seo JY, et al. Bone cement augmentation procedures for spinal pathologic fractures by multiple myeloma. J Korean Med Sci. 2015; 30(1):88–94.

5. Schuster JM, Grady MS. Medical management and adjuvant therapies in spinal metastatic disease. Neurosurg Focus. 2001; 11(6):e3.

6. Lim BS, Chang UK, Youn SM. Clinical outcomes after percutaneous vertebroplasty for pathologic compression fractures in osteolytic metastatic spinal disease. J Korean Neurosurg Soc. 2009; 45(6):369–374.

7. Chong S, Shin SH, Yoo H, et al. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients' survival. Spine J. 2012; 12(12):1083–1092.

8. Hirabayashi H, Ebara S, Kinoshita T, et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003; 97(2):476–484.

9. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008; 8(3):271–278.

10. Yamashita T, Aota Y, Kushida K, et al. Changes in physical function after palliative surgery for metastatic spinal tumor: association of the revised Tokuhashi score with neurologic recovery. Spine (Phila Pa 1976). 2008; 33(21):2341–2346.

11. Kwon YM, Kim KS, Kuh SU, Chin DK, Jin BH, Cho YE. Survival rate and neurological outcome after operation for advanced spinal metastasis (Tomita's classification > or = type 4). Yonsei Med J. 2009; 50(5):689–696.

12. Fujibayashi S, Neo M, Miyaki K, Nakayama T, Nakamura T. The value of palliative surgery for metastatic spinal disease: satisfaction of patients and their families. Spine J. 2010; 10(1):42–49.

13. Dea N, Versteeg A, Fisher C, et al. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine. 2014; 21(5):698–703.

14. Rao PJ, Thayaparan GK, Fairhall JM, Mobbs RJ. Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop Surg. 2014; 6(3):187–195.

15. Kim CH, Chung CK, Sohn S, Lee S, Park SB. Less invasive palliative surgery for spinal metastases. J Surg Oncol. 2013; 108(7):499–503.

16. Lau D, Leach MR, La Marca F, Park P. Independent predictors of survival and the impact of repeat surgery in patients undergoing surgical treatment of spinal metastasis. J Neurosurg Spine. 2012; 17(6):565–576.

17. Hunter GK, Balagamwala EH, Koyfman SA, et al. The efficacy of external beam radiotherapy and stereotactic body radiotherapy for painful spinal metastases from renal cell carcinoma. Pract Radiat Oncol. 2012; 2(4):e95–e100.

18. Wu JS, Wong RK, Lloyd NS, et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases: an evidence-based practice guideline. BMC Cancer. 2004; 4:71.
19. Schlampp I, Rieken S, Habermehl D, et al. Stability of spinal bone metastases in breast cancer after radiotherapy: a retrospective analysis of 157 cases. Strahlenther Onkol. 2014; 190(9):792–797.

20. Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015; 22(4):409–415.

21. Lee S, Chun M. Pain relief by Cyberknife radiosurgery for spinal metastasis. Tumori. 2012; 98(2):238–242.

22. Farrokhi M, Nouraei H, Kiani A. The efficacy of percutaneous vertebroplasty in pain relief in patients with pathological vertebral fractures due to metastatic spinal tumors. Iran Red Crescent Med J. 2012; 14(9):523–530.
23. Cheung G, Chow E, Holden L, et al. Percutaneous vertebroplasty in patients with intractable pain from osteoporotic or metastatic fractures: a prospective study using quality-of-life assessment. Can Assoc Radiol J. 2006; 57(1):13–21.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download