Abstract
Background
The purpose of this study was to analyze the relation between intramedullary high signal intensity (IMHS) on magnetic resonance imaging (MRI), radiographic parameters, and clinical symptoms in cervical ossification of the posterior longitudinal ligament (OPLL) patients.
Methods
Two hundred forty-one patients, who underwent simple radiography, computed tomography (CT), and MRI were included in the present study. As radiographic parameters, the OPLL occupying ratio and occupying area were measured on CT images. Dynamic factors were assessed by measuring cervical range of motion (ROM) on simple radiographs. Visual analog scale (VAS) for neck and arm pain, and Japanese Orthopaedic Association (JOA) scores were evaluated for clinical analysis. The differences in radiographic and clinical findings were assessed between patients with IMHS on T2-weighted MRI findings (group A) and patients without IMHS (group B).
Results
Eighty-one patients were assigned to group A and 160 patients to group B. The occupying ratios were found to be higher in group A than in group B on both sagittal and axial views (p < 0.01). Group A also showed a higher area occupying ratio (p < 0.01). The length and area of underlying spinal canal on the sagittal and cross-sectional planes were lower in group A than in group B (p < 0.01). No significant difference in ROM was observed (p = 0.63). On the clinical findings, group A had a lower JOA score (p < 0.001), and no intergroup differences in VAS scores were observed.
Conclusions
In cervical OPLL cases, IMHS on MRI was associated with manifestation of myelopathic symptom. Occupying ratio was associated with high signal intensity on MRI, whereas no association was found with ROM. Occurrence of high signal intensity increased inversely with the length and area of underlying spinal canal.
Ossification of the posterior longitudinal ligament (OPLL) is a condition of abnormal calcification in the posterior longitudinal ligament, and mostly involves the cervical spine. Compression of the spinal cord by the OPLL mass can lead to neurological symptoms, and often operative treatments are required in the cases of severe neurological deficit.1) Despite a high degree of spinal cord compression, symptoms do not manifest in many cases, because of which physicians often face the dilemma of choosing the most appropriate treatment strategy such as prophylactic surgery,2) early surgical intervention,3) or conservative treatment in mild symptomatic patients.4) In particular, unlike cervical spondylotic myelopathy, OPLL often fails to manifest clinical symptoms despite severely compressed radiographic findings, and sometimes goes undetected life-long.
Magnetic resonance imaging (MRI) is a useful radiographic method for the diagnosis of cervical myelopathy that evaluates the status of spinal stenosis and intramedullary condition.5) In particular, intramedullary high signal intensity (IMHS) on T2-weighted MRI has been reported to be indicative of changes in spinal gray matter,6) associated with higher severity of myelopathy symptoms,7) and negative postoperative prognostic factor.8) Nevertheless, not all OPLL cases with myelopathy show high signal intensity on MRI, and there are even cases where patients with high signal intensity findings exhibit no symptoms of myelopathy.
A large number of previous studies have investigated the relevance of high signal intensity on MRI to symptoms of cervical spondylotic myelopathy and its prognosis, and there is a lack of research on the significance of IMHS on MRI in OPLL patients in relation to clinical symptoms. To address this issue, we have analyzed the interrelations between IMHS on MRI, radiographic parameters, and clinical symptoms in patients with cervical OPLL.
Among 365 cervical OPLL patients from Inje University Haeundae Paik Hospital between 2010 and 2012, we selected 241 patients (146 men and 95 women; mean age, 52.4 years) as subjects for analysis in this study. The inclusion criteria were as follows: (1) diagnosis of cervical OPLL; and (2) patients who underwent simple radiography, computed tomography (CT) scan and MRI. The exclusion criteria were history of trauma and prior history of cervical operation. If the radiological evaluations of CT or MRI were performed at different qualifications in another hospital, such cases were excluded from analysis. Institutional Review Board approval was obtained for this study. The patient distribution according to OPLL type was as follows: local (n = 32), segmental (n = 79), mixed (n = 102), and continuous (n = 28).
High signal intensity was defined as a case showing high signal intensity in both sagittal and cross-sectional planes on T2-weighted MRI scan as assessed by a radiologist. The occupying ratio (the ratio of the length occupied by the OPLL to the normal spinal canal length) was determined (Fig. 1A and B) by measuring the length available for the spinal cord and the length of the unaffected spinal canal on sagittal and axial view on the CT scan in maximal compression area. The area occupying ratio (area occupied by the OPLL/area of the normal spinal cord) of the cross-section with the highest degree of cord compression was also determined (Fig. 1C).9) The entire measurement of CT scan was done using the axial cut which was made parallel to disc space. For the assessment of dynamic factors, we measured the cervical range of motion (ROM) by determining the differences of the extension lines from the lower edge of the second cervical vertebra and the lower edge of the seventh cervical vertebra, respectively, on simple flexion and extension radiographs (Fig. 1D). All the measurements were performed by the PACS system (m-view; Maro Tech Inc., Seoul, Korea). Two blinded observers independently interpreted the radiological findings twice and the mean values were used for further measurements. In order to verify the reliability of the measured values, the intra- and interobserver correlations were checked using intra- and interclass correlation coefficient (ICC).10) ICC value (Cronbach α) was analyzed by standardized confidence analysis and categorized as following: poor (α < 0.4), fair to good (0.4 to 0.7), and excellent (α > 0.7).
Clinical analysis was performed by using visual analog scale (VAS) for neck and arm pain, and Japanese Orthopaedic Association (JOA) scores. Differences in clinical features were determined by comparing radiographic and clinical findings between patients showing high signal intensity on T2-weighted MRI findings (group A) and those who did not show high signal intensity (group B).
Statistical analysis included t-test and chi-square tests using the SPSS ver. 10.1 (SPSS Inc., Chicago, IL, USA).
The ICC for intra- and interobserver reliability showed that the data used for this study were reliable (0.63 and 0.62, respectively).
Of the 241 enrolled patients, 81 (33.6%) were assigned to group A and 160 were assigned to group B (Table 1). Group A had an older mean age (58.25 vs. 53.38 years; p < 0.001), and the men outnumbered the women with respect to occurrence of high signal intensity (group A, 75% and group B, 53%; p < 0.001). The occupying ratios determined by radiographic analysis were found to be higher in group A than in group B on both sagittal and axial planes (49.45% and 49.96% vs. 38.77% and 38.27%; p < 0.01) (Table 2). Group A also showed a higher area occupying ratio with statistical significance (32.86% vs. 22.69%; p < 0.01).
The length and area of the unaffected spinal canal on the sagittal and axial planes were measured to be statistically lower in group A (11.05 mm, 11.91 mm, and 208.27 mm2; p < 0.01) than in group B (11.87 mm, 12.77 mm, and 228.93 mm2; p < 0.01). Among the OPLL types, the mixed type was found to be most frequent in group A (p < 0.001) (Table 3).
No significant difference was seen in ROM (group A, 25.47° and group B, 25.78°; p = 0.63). As clinical conditions, the mean JOA score was 15.13 ± 2.52, and the VAS scores for neck pain and arm pain were 3.4 ± 2.50 and 3.91 ± 2.90, respectively. Group A showed a lower JOA score (13.53, p < 0.001), and no intergroup difference in neck and arm VAS was observed (Table 1).
MRI is a useful tool for diagnosing cervical myelopathy, which reveals not only the degree of spinal cord compression but also intramedullary conditions in detail.5) Since Takahashi et al.11)'s report on the IMHS on MRI in cervical spondylotic myelopathy, findings of low and high signal intensities on T1- and T2-weighted MRI, respectively, have been reported as frequent findings in severe myelopathy and indicate poor postoperative prognosis.78) In the case of myelopathy patients, who need surgical treatment, good clinical outcomes can be expected with timely surgical intervention before development of intramedullary signal change on MRI. So, it is essential to analyze the risk factors for development of high signal intensity in MRI. Early surgical intervention can be recommended to the patients demonstrating risk factors; thus, poor clinical outcomes from delay in surgical treatment can be prevented. Wang et al.12) reported that high signal intensity on T2-weighted MRI and pyramidal sign in patients with cervical OPLL are associated with reduced ability to recover from spinal cord damage and poor postoperative prognosis. Qizhi et al.9) identified long-term symptoms, high occupying ratios, low preoperative JOA scores, kyphosis, and cervical instability as factors related to IMHS in patients with OPLL and recommended early surgical treatment to patients with these factors. In the present study, intramedullary signal change was more frequently present in older patients and men. In radiographic findings, high signal intensity increased in inverse proportion to the length and area of underlying spinal canal diameters on sagittal and cross-sectional planes. The OPLL occupying ratio, a static factor, was found to be associated with high signal intensity on MRI, but no association was confirmed with regards to ROM, a dynamic factor. Matsunaga et al.13) reported that in 156 cervical OPLL patients with a mean follow-up duration of 10.3 years, myelopathy occurred in all cases exhibiting 60% spinal stenosis or higher and in 49% of cases exhibiting less than 60% spinal stenosis, with aggravating tendency in large ROM and laterally tilted shape. Mochizuki et al.7) reported that myelopathy symptoms were more frequent in cases of large ROM and segmental OPLL. In the present study, however, ROM was found to have no significant relation with myelopathic symptoms, and the area-related degree of stenosis showed a statistically significant association. In our opinion, these results are ascribed to the findings that the degree of compression in OPLL was influenced more by the occurrence of high signal intensity than by cervical ROM. Most cases of continuous or mixed OPLL showed severe compression and narrow cervical ROM. Among previous studies, Chang et al.14) also reported that myelopathy symptoms in patients with OPLL were not related to ROM and tended to occur in accordance with the degree of maximum spinal cord compression. For more accurate determination as to which factor is more related to the occurrence of myelopathy symptoms, further evaluation on the degree of compression, ROM, and findings of IMHS is needed.
Apart from the unsettled discussion about prophylactic and early-phase surgery, IMHS can serve as an indication for timely surgery to prevent further damage to the spinal cord. This aspect will have to be explored in further studies.
The limitation of this study includes its retrospective design. Furthermore, evaluation according to other diverse radiological factors on MRI such as signal change on T1-weighted image or length of signal change was not evaluated. However, we evaluated a relatively large number of OPLL patients and this result can serve as useful clinical information in proper management of cervical OPLL patients.
Finally in cervical OPLL, IMHS on T2-weighted MRI was associated with the manifestation of symptoms of cervical myelopathy. The high signal intensity was found to be associated with the occupying ratio, a static factor, but was not associated with ROM, a dynamic factor of OPLL. Moreover, occurrence of high signal intensity increased in inverse proportion to the length and area of underlying spinal canal stenosis on sagittal and cross-sectional planes.
Figures and Tables
Fig. 1
Radiological evaluations. (A) Sagittal occupying ratio: (b-a)/b. a: sagittal space available for cord, b: sagittal canal length. (B) Axial occupying ratio: (d-c)/d. c: axial space available for cord, d: axial canal length. (C) Area occupying ratio: f/e. e: area of spinal canal, f: area of ossification of posterior longitudinal ligament. (D) Range of motion (yellow line: endplate of C2 and C7).
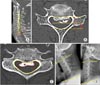
Table 1
Demographic and Clinical Characteristics of Two Groups

Table 2
Difference of Radiologic Parameters between Two Groups

References
1. Jeon TS, Chang H, Choi BW. Analysis of demographics, clinical, and radiographical findings of ossification of posterior longitudinal ligament of the cervical spine in 146 Korean patients. Spine (Phila Pa 1976). 2012; 37(24):E1498–E1503.

2. Epstein N. Diagnosis and surgical management of cervical ossification of the posterior longitudinal ligament. Spine J. 2002; 2(6):436–449.

3. Ogawa Y, Chiba K, Matsumoto M, et al. Long-term results after expansive open-door laminoplasty for the segmental-type of ossification of the posterior longitudinal ligament of the cervical spine: a comparison with nonsegmental-type lesions. J Neurosurg Spine. 2005; 3(3):198–204.

4. Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: does it predict the outcome of conservative treatment? Spine (Phila Pa 1976). 2000; 25(6):677–682.

5. Ohshio I, Hatayama A, Kaneda K, Takahara M, Nagashima K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976). 1993; 18(9):1140–1149.

6. Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical myelopathy: analysis of 29 surgical cases. J Neurosurg. 1991; 74(6):887–892.
7. Mochizuki M, Aiba A, Hashimoto M, Fujiyoshi T, Yamazaki M. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2009; 10(2):122–128.

8. Sun Q, Hu H, Zhang Y, et al. Do intramedullary spinal cord changes in signal intensity on MRI affect surgical opportunity and approach for cervical myelopathy due to ossification of the posterior longitudinal ligament? Eur Spine J. 2011; 20(9):1466–1473.

9. Qizhi S, Lili Y, Ce W, Yu C, Wen Y. Factors associated with intramedullary MRI abnormalities in patients with ossification of the posterior longitudinal ligament. J Spinal Disord Tech. 2015; 28(5):E304–E309.

10. Henriksen M, Lund H, Moe-Nilssen R, Bliddal H, Danneskiod-Samsoe B. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture. 2004; 19(3):288–297.

11. Takahashi M, Sakamoto Y, Miyawaki M, Bussaka H. Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology. 1987; 29(6):550–556.

12. Wang LF, Zhang YZ, Shen Y, et al. Using the T2-weighted magnetic resonance imaging signal intensity ratio and clinical manifestations to assess the prognosis of patients with cervical ossification of the posterior longitudinal ligament. J Neurosurg Spine. 2010; 13(3):319–323.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download