Abstract
Background
Wear cannot be completely prevented after total hip arthroplasty. If severe polyethylene (PE) liner wear develops, the so-called catastrophic failure occurs and metallosis develops. We postulated that longevity of the new implant may be affected after revision surgery for metallosis following a catastrophic failure of a PE liner due to the substantial amount of PE wear particles and infiltration of the metal particles in this catastrophic condition.
Methods
Twenty-three hips of 23 patients were identified because they showed metallosis during revision total hip arthroplasties performed in Seoul National University Hospital between January 1996 and August 2004. They were followed for at least 6.5 years after the index revision total hip arthroplasty. The clinical and radiological results of revision total hip arthroplasties in these patients were evaluated.
Results
The median Harris hip score increased from 60 points before revision total hip arthroplasties to 90 points at the final follow-up. Osteolysis was detected at an average of 9.3 years after revision total hip arthroplasties in 13 hips and acetabular cup loosening at average 9.8 years after revision total hip arthroplasties in 9 hips. With radiographic evidence of osteolysis and loosening as the end points, the 15-year survival rates were 28.2% and 56.0%, respectively.
Total hip arthroplasty (THA) is an effective treatment for various hip diseases that do not respond to conservative or other operative management.1) However, there may be substantial complications after THA.2,3,4) Among them, wear cannot be completely prevented by any treatment method.5,6) If severe polyethylene (PE) liner wear develops, the so-called catastrophic failure occurs and the head component could come in contact with the acetabular metal shell. This condition causes unexpected friction between the head and acetabular components. If either of these two components is made of a metal, metal wear particles will be generated in addition to PE wear particles, and thus, 'metallosis' develops.7,8,9) Metallosis is a term used to describe the infiltration of metallic wear debris into the periprosthetic structures, including soft and bony tissues.7) Wear particles provoke an immunologic reaction, leading to osteolysis followed by aseptic implant loosening.10) Osteolysis in the presence or absence of aseptic loosening may require reoperation or revision THA.11)
We postulated that the longevity of the new implant may be affected after revision surgery for metallosis following a catastrophic failure of a PE liner because of the substantial amount of PE wear particles and infiltration of the metal particles in this catastrophic condition.
In this study, we evaluated the clinical characteristics and the survival rate of revision THA performed in patients with metallosis following a catastrophic failure of a PE liner. This study addresses two research questions. First, does metallosis following a catastrophic failure of a PE liner affect the results of revision THA? Second, is there a reliable factor for predicting the results of revision THA in patients with metallosis?
We identified 27 hips in 27 patients who underwent revision THA and showed metallosis from a catastrophic failure of a PE liner in their hip joints during revision THA among all the patients who underwent revision THAs in Seoul National University Hospital between January 1996 and August 2004. One patient died 7 days after revision THA due to cardiac arrest. Three patients were lost to follow-up during the 5 years period after surgery and could not be reached by telephone or mail. Excluding these 4 patients, 23 patients (12 men and 11 women) had THA at the average age of 38.4 years (range, 15 to 63 years), had index revision THAs at the average age of 48.1 years (range, 23 to 78 years) and the average interval between THA and revision THA was 9.7 years (range, 4.0 to 14.2 years). Patients were followed for at least 6.5 years after the index revision THA (average duration of follow-up, 12.5 years; range, 6.5 to 18.0 years). Nineteen of the 23 hips underwent clinical and radiographic evaluations and four hips underwent clinical evaluation only, which was conducted over the telephone and with a questionnaire sent by mail.
The initial cause of primary THA was osteonecrosis of the femoral head in 7 patients, degenerative arthritis in 5 patients, Legg-Calve-Perthes disease sequelae in 3 patients, developmental dysplasia of the hip in 2 patients, infection sequelae in 2 patients, tuberculosis sequelae in 1 patient, developmental coxa valga in 1 patient, dysplasia in 1 patient, and rheumatoid arthritis in 1 patient. Metal-on-PE bearings were used for primary THA in 22 patients, and ceramic-on-PE bearing was used in 1 patient. Nineteen THAs were done in our hospital and their implants were the AML hip system (DePuy, Warsaw, IN, USA) in 8 hips and Osteonics hip system (Stryker, Kalamazoo, MI, USA) in 11 hips. Liners of these 19 THAs were conventional PE. Implants of remaining 4 hips are not identifiable because the THAs were performed at other hospitals and the corresponding medical records were insufficient. Considering the time of the primary THAs (1988, 1994, 1995, and 1996), the unidentifiable 4 liners probably were conventional PE.
The revision THAs were performed due to osteolysis without loosening in 21 hips and osteolysis with loosening of the cup in 2 hips. Two hip surgeons who had more than 15 years of experience with hip operations each, performed all the revision THAs. The bubble sign, which is known to be related with metallosis, was observed in 10 hips on plain radiographs taken before revision THAs.12) The extent and location of the osteolysis were evaluated on preoperative radiographs, and bone deficiency around the cup and stem was categorized according to the system of the American Association of Orthopaedic Surgeons.13,14) Osteolysis was defined as periprosthetic cystic or scalloped lesions with a diameter exceeding 2 mm that had not been noted on the immediate postoperative radiograph.15,16) Among the 23 hips, 15 hips showed type III acetabular defects. Type II acetabular defects were observed in 7 hips, and a type I acetabular defect was observed in one hip. Osteolysis around the femur was also observed in all 23 hips before revision THAs with type I osteolysis in 6 hips and type II osteolysis in 17 hips. Total revision and cup revision procedures were performed in 13 and 10 hips, respectively. Posterolateral and transtrochanteric approaches were used for revision THAs in 10 hips and in 13 hips, respectively. Cementless acetabular implants were used in 22 hips and an acetabular reinforcement ring was used in one hip for revision THAs. Chang et al.7) classified the severity of metallosis as grade I (mild: black spotting in the soft tissues), grade II (moderate: geographically patterned black stain in soft tissues), and grade III (severe: black spotting throughout the soft tissues and bones). We used the same grading criteria and all 23 hips in this study showed grade III metallosis (Fig. 1) during revision THAs. We irrigated the operative field copiously and removed the functionless black-stained tissue as best we could. Bone grafts were applied to both the acetabular and femoral defects in all hips. During revision surgery, metal-on-PE bearings were used in 12 hips, ceramic-on-ceramic bearings were used in 5 hips, ceramic-on-PE bearings were used in 4 hips, and metal-on-metal bearings were used in 2 hips. Three of 16 PE liners were crossed linked PE and the others were conventional PE.
Clinical evaluations were performed using Harris hip scores.17) When the patient could not visit the hospital, the evaluation was completed via telephone or by mail. Serial anteroposterior radiologic images were obtained to evaluate the presence of osteolysis or loosening. Radiographs were evaluated by two independent observers, who had completed their residency and fellowship programs and were working as adult hip reconstruction specialists for 22 years and 5 years, respectively, and were well-experienced in assessing the presence of osteolysis and loosening. A change in the angle of more than 4°, or vertical or horizontal migration of more than 3 mm were taken as evidences of an unstable cup.15) Instability of the femoral stem was defined as subsidence or vertical migration of more than 2 mm or a change in the stem angle of more than 2°, on the anteroposterior radiographs.18)
Kaplan-Meier survival analysis was performed to assess the implant survival after revision. The 15-year survival rates of revision THA were measured using osteolysis or loosening as the endpoint, respectively. Demographic and clinical variables were compared by using chi-square or Fisher exact tests for categorical variables such as gender, bubble sign, revision type, bearing for revision THA and bony defect severity between patients demonstrating osteolysis or loosening and the others. The t-test was also used to compare continuous variables such as age at revision THA and body mass index (BMI). The level of significance was set at p < 0.05.
Two patients died during the study period. They both died of an unrelated medical condition at 6.5 years and 16 years after their revision THA procedures, respectively. One of these two patients could not visit the outpatient clinic due to a geographical reason and did not complain of any hip problems over the phone before death. The other patient died 16 years after the revision THA and used a cane due to hemiplegia from a cerebrovascular attack.
The median Harris hip score increased from 60 points (range, 35 to 89 points) before revision THA to 90 points (range, 3 to 98 points) at the final follow-up (Fig. 2). Three patients reported a decrease in the Harris hip score at the last follow-up. All of the patients underwent re-revision THAs for osteolysis with loosening.
Osteolysis was detected on average 9.3 years (range, 5.3 to 13.5 years) after revision THAs in 13 hips (Fig. 3). Among them, the acetabular cup was loosened an average of 9.8 years (range, 5.7 to 18.3 years) after revision THA in 9 hips. Re-revision of the cup was performed in 5 hips and re-revision of both the cup and stem was performed in 2 of the 9 loosened hips (Fig. 4). In one hip with cup re-revision, cup removal and cutting of the artificial femoral neck were performed due to severe bone loss and vessel injury following cup re-revision due to infection on two occasions. Among the other two patients with loosening, one patient could perform his daily activities with a cane and refused to undergo re-revision surgery. The other patient was recently recommended revision THA, but did not undergo the procedure under the time constraints of this study.
One of the 4 patients in whom osteolysis without loosening was detected underwent a head and liner change with allogeneic bone grafting into the acetabular bone defect. The remaining 3 patients who showed osteolysis around the well-fixed implant did not complain of any discomfort and were followed-up without any further operations. Clinical and radiographic data are summarized in Table 1 and Fig. 4.
With radiographic evidence of osteolysis and loosening as the end points, the 15-year survival rate was 28.2% (95% confidence interval [CI], 8.3 to 52.6) and 56.0% (95% CI, 27.3 to 77.2), respectively (Fig. 5).
Statistical analysis did not reveal that any variables among age at revision THA, gender, BMI, bony defect severity, bubble sign, bearings used for revision THA, and revision type had a significant association with osteolysis or loosening after revision THA.
Metallosis from a catastrophic failure of a PE liner is a devastating condition. During our study period, the revision rate was 30.4% after revision THA, which was very high compared to the re-revision rate of other recent studies reporting the results of revision THA in the presence or absence of metallosis.19,20) Moreover, the incidences of osteolysis and loosening after revision THA in this study were 56.5% and 39.1%, respectively, and they were also higher than those in other studies.20)
This study has some limitations that need to be mentioned. First, there was no control group that included patients who had only catastrophic liner wear without metallosis, for comparing and determining the exact effect of metallosis on the results of revision THA. However, this study demonstrated that severe metallosis resulting from catastrophic liner wear by itself is a risk factor for the failure of revision THA. Second, we could not identify any factors which affect the results of revision THA following severe metallosis. Twenty-three cases do not constitute an adequate sample size to allow for analysis of other factors that may affect the results of revision THA. Third, the extent of metallosis or volume of metal wear was not evaluated. Quantification of metallosis or metal wear might give further information on the volumetric effect of the metal problem. In fact, it is difficult to define the margin of metallosis because there is grossly invisible metallic invasion.
During revision THA, all of the hips showed severe metallosis classified as grade III in this study. Bony defects before revision THA were also severe, and acetabular defects were noted in all 23 hips. In contrast, Chang et al.7) reported osteolysis around the acetabular cup in only 24 of 31 hips (77.4%) demonstrating metallosis, and only 4 of these hips showed a type III acetabular defect. Their results were not in agreement with those of the current study, in which 15 of 23 hips showed type III acetabular bony defect.14) This may be related to the mechanism of metallosis. It is known that liner dissociation, catastrophic liner wear, or direct impingement between components, such as shell and stem or shell and screw, can cause metallosis.7,12) Among them, PE liner wear usually does not cause symptoms that are different from those of liner dissociation or restricted motion due to impingement. Metallosis from catastrophic liner wear can continue to progress, generating many PE and metal wear particles. PE wear particles are known to cause an inflammatory reaction and subsequent osteolysis.21) Moreover, metal wear particles and PE wear particles can exert a synergistic effect.10) Metallosis due to catastrophic liner wear leads to osteolysis and loosening.
The problems caused by metal wear particles have been identified recently due to failed metal-on-metal THA and resurfacing THA.22,23,24,25) Pseudotumor is one such problem. Pseudotumor was first described as a soft-tissue mass associated with the metal-on-metal resurfacing implant, and it is neither malignant nor infective in nature.25) Substantial necrosis and a heavy macrophage infiltrate were noted around the pseudotumor. The adaptive immune response also plays an important role in the development of pseudotumor.23) Direct cytotoxicity of metal particles and immunologic reaction play an important role in problems related to metal ions.22,24) The increased incidence of osteolysis after revision THA following severe metallosis could be partially explained by the same phenomenon.
In conclusion, the survival rate of revision THA in patients with metallosis following a catastrophic failure of a PE liner was found to be low. A substantial amount of PE wear debris and the infiltration of metallic wear particles in the periprosthetic tissues might lead to progressive bone loss and implant loosening, even after revision THA. The patient, in whom this catastrophic condition is detected during revision surgery, should be informed that he/she has to regularly attend follow-up visits due to a high possibility of additional revision surgeries.
Figures and Tables
 | Fig. 1Metallosis following catastrophic failure of polyethylene liner. (A) Grade III metallosis was observed around the hip joint during revision total hip arthroplasty (THA). (B) A photomicrograph of a specimen taken during revision THA shows histiocytic infiltration with abundant metallic debris (H&E, ×400). |
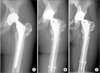 | Fig. 2(A) Catastrophic polyethylene wear and osteolysis were observed 10 years after primary total hip arthroplasty (THA). (B) Revision THA was performed using a ceramic-on-ceramic bearing. (C) There was no osteolysis or loosening 11 years after revision THA. |
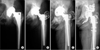 | Fig. 3(A) Catastrophic polyethylene (PE) wear and osteolysis were observed 8 years after primary total hip arthroplasty (THA). (B) Revision THA was performed using a ceramic head and a PE liner. Due to loosening of the acetabular cup (C), re-revision THA was performed 9 years after revision THA (D). |
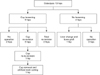 | Fig. 4Flowchart demonstrating the clinical and radiographic results of 13 hips with osteolysis after revision total hip arthroplasty following metallosis from catastrophic liner wear. |
Table 1
Demographic, Clinical and Radiographic Data of 23 Hips with Metallosis from Catastrophic Wear

References
1. Yoon PW, Kim JI, Kim DO, et al. Cementless total hip arthroplasty for patients with Crowe type III or IV developmental dysplasia of the hip: two-stage total hip arthroplasty following skeletal traction after soft tissue release for irreducible hips. Clin Orthop Surg. 2013; 5(3):167–173.

2. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004; (429):188–192.
3. Nam KW, Yoo JJ, Kim YL, Kim YM, Lee MH, Kim HJ. Alumina-debris-induced osteolysis in contemporary aluminaon-alumina total hip arthroplasty: a case report. J Bone Joint Surg Am. 2007; 89(11):2499–2503.

4. Park SK, Kim YG, Kim SY. Treatment of periprosthetic femoral fractures in hip arthroplasty. Clin Orthop Surg. 2011; 3(2):101–106.

5. Elsner JJ, Shemesh M, Mezape Y, et al. Long-term evaluation of a compliant cushion form acetabular bearing for hip joint replacement: a 20 million cycles wear simulation. J Orthop Res. 2011; 29(12):1859–1866.

6. Esposito CI, Walter WL, Roques A, et al. Wear in aluminaon-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012; 94(7):901–907.
7. Chang JD, Lee SS, Hur M, Seo EM, Chung YK, Lee CJ. Revision total hip arthroplasty in hip joints with metallosis: a single-center experience with 31 cases. J Arthroplasty. 2005; 20(5):568–573.
8. Donaldson JR, Miles J, Sri-Ram K, Poullis C, Muirhead-Allwood S, Skinner J. The relationship between the presence of metallosis and massive infection in metal-on-metal hip replacements. Hip Int. 2010; 20(2):242–247.

9. Khan RJ, Wimhurst J, Foroughi S, Toms A. The natural history of metallosis from catastrophic failure of a polyethylene liner in a total hip. J Arthroplasty. 2009; 24(7):1144.e1–1144.e4.

10. Wooley PH, Morren R, Andary J, et al. Inflammatory responses to orthopaedic biomaterials in the murine air pouch. Biomaterials. 2002; 23(2):517–526.

11. Spicka J, Gallo J, Cechova I, Kaminek P, Mikulik J. Surgical therapy of osteolysis around stable cementless hip arthroplasty. Acta Chir Orthop Traumatol Cech. 2005; 72(4):228–234.
12. Su EP, Callander PW, Salvati EA. The bubble sign: a new radiographic sign in total hip arthroplasty. J Arthroplasty. 2003; 18(1):110–112.
13. D'Antonio J, McCarthy JC, Bargar WL, et al. Classification of femoral abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1993; (296):133–139.
14. D'Antonio JA, Capello WN, Borden LS, et al. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989; (243):126–137.
15. Joshi RP, Eftekhar NS, McMahon DJ, Nercessian OA. Osteolysis after Charnley primary low-friction arthroplasty: a comparison of two matched paired groups. J Bone Joint Surg Br. 1998; 80(4):585–590.
16. Maloney WJ, Jasty M, Harris WH, Galante JO, Callaghan JJ. Endosteal erosion in association with stable uncemented femoral components. J Bone Joint Surg Am. 1990; 72(7):1025–1034.

17. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969; 51(4):737–755.

18. Martell JM, Pierson RH 3rd, Jacobs JJ, Rosenberg AG, Maley M, Galante JO. Primary total hip reconstruction with a titanium fiber-coated prosthesis inserted without cement. J Bone Joint Surg Am. 1993; 75(4):554–571.

19. Schmitz MW, Busch VJ, Gardeniers JW, Hendriks JC, Veth RP, Schreurs BW. Long-term results of cemented total hip arthroplasty in patients younger than 30 years and the outcome of subsequent revisions. BMC Musculoskelet Disord. 2013; 14:37.
20. Yoo JJ, Yoon PW, Lee YK, Koo KH, Yoon KS, Kim HJ. Revision total hip arthroplasty using an alumina-on-alumina bearing surface in patients with osteolysis. J Arthroplasty. 2013; 28(1):132–138.

21. Skoglund B, Aspenberg P. PMMA particles and pressure: a study of the osteolytic properties of two agents proposed to cause prosthetic loosening. J Orthop Res. 2003; 21(2):196–201.

22. Campbell P, Shimmin A, Walter L, Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008; 23(7):1080–1085.

23. Grammatopoulos G, Pandit H, Kamali A, et al. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013; 95(12):e81.

24. Kwon YM, Xia Z, Glyn-Jones S, Beard D, Gill HS, Murray DW. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater. 2009; 4(2):025018.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download