Abstract
Background
Distraction osteogenesis (DO) is a promising tool for bone and tissue regeneration. However, prolonged healing time remains a major problem. Various materials including cells, cytokines, and growth factors have been used in an attempt to enhance bone formation. We examined the effect of percutaneous injection of demineralized bone matrix (DBM) during the consolidation phase on bone regeneration after distraction.
Methods
The immature rabbit tibial DO model (20 mm length-gain) was used. Twenty-eight animals received DBM 100 mg percutaneously at the end of distraction. Another 22 animals were left without further procedure (control). Plain radiographs were taken every week. Postmortem bone dual-energy X-ray absorptiometry and micro-computed tomography (micro-CT) studies were performed at the third and sixth weeks of the consolidation period and histological analysis was performed.
Results
The regenerate bone mineral density was higher in the DBM group when compared with that in the saline injection control group at the third week postdistraction. Quantitative analysis using micro-CT revealed larger trabecular bone volume, higher trabecular number, and less trabecular separation in the DBM group than in the saline injection control group. Cross-sectional area and cortical thickness at the sixth week postdistraction, assessed using micro-CT, were greater in the regenerates of the DBM group compared with the control group. Histological evaluation revealed higher trabecular bone volume and trabecular number in the regenerate of the DBM group. New bone formation was apparently enhanced, via endochondral ossification, at the site and in the vicinity of the injected DBM. DBM was absorbed slowly, but it remained until the sixth postoperative week after injection.
Conclusions
DBM administration into the distraction gap at the end of the distraction period resulted in a significantly greater regenerate bone area, trabecular number, and cortical thickness in the rabbit tibial DO model. These data suggest that percutaneous DBM administration at the end of the distraction period or in the early consolidation period may stimulate regenerate bone formation and consolidation in a clinical situation with delayed bone healing during DO.
Distraction osteogenesis (DO) is a surgical technique in which new bone formation is induced by gradual distraction of a fracture callus after low-energy corticotomy, with careful preservation of the soft tissue surrounding the bone.1) DO is primarily used in various difficult or intractable clinical conditions such as chronic osteomyelitis,2) critical bone defect,3) severe bone deformity, and leg length discrepancy,45) because of its powerful potential to regenerate bone.
In spite of its benefits and potential as a limb reconstruction tool, prolonged healing time remains a major problem. The new bone formed at the distraction site generally requires a much longer consolidation period than distraction period, resulting in a bone healing index (time in external fixation/length gained) of more than 30 days per centimeter of adult limb lengthening.678) A long duration of external fixation for distracting and stabilizing the osteotomy site increases the complications and causes inconvenience to the patients.9)
Recent clinical and experimental research has been focused on developing methods to shorten the duration of DO in order to reduce several problems.10) Since the biological mechanism of DO has been studied deeply, several materials have been investigated to reduce the duration of DO. Enhancement of bone formation has been attempted using various materials including mesenchymal stem cells,1112) bone morphogenetic protein (BMP),131415) fibroblast growth factor,16) prostaglandin E receptor agonist,17) and bone conduction agents such as calcium sulfate.18) However, these materials have limitations in terms of accessibility, high cost, short duration of effect and/or low effectiveness.
Demineralized bone matrix (DBM), which was first introduced by Van de Putte and Urist,19) contains glycoproteins and collagen which are expected to possess both osteoinductive and osteoconductive properties.202122) The efficacy of DBM has been proved clinically and various types of DBM are commercially available.23) Hagino and Hamada24) reported that applying DBM in the rabbit shortened the duration of DO, indicating the possibility that DBM enhances bone healing during DO.
In this study, we attempted to assess the effect of percutaneous injection of DBM during the consolidation phase on bone regeneration after distraction. We chose to inject DBM at the end of distraction at a normal speed (1 mm per day) for clinical relevance. The purposes of this study were (1) to assess the effect of percutaneous injection of DBM on bone regeneration after distraction, (2) to prove that the end of the distraction period may be the appropriate timing for DBM injection, and (3) to reveal the mechanism of enhanced bone regeneration after the injection of DBM.
All animal studies were performed with approval from the Animal Care Committee at Seoul National University Hospital. Fifty 10-week-old male New Zealand white rabbits (2.0-2.5 kg) underwent right-sided tibial lengthening. After premedication with intramuscular ketamine (50 mg/kg) and xylazine (10 mg/kg), anesthesia was administered with intravenous Zoletil (Virbac, Carros, France). After preparation of the right lower extremity, an open midtibial osteotomy was performed using oscillating saw and osteotome, and two monofixators (Solco, Seoul, Korea) were applied bilaterally, using four 0.029 inch Kirschner wires (Solco). The periosteum was carefully closed with a 4-0 vicryl suture. The left lower extremity was left intact. Subcutaneous injection of buprenorphine (0.1 mg/kg) was administered at the end of surgery and again at 12 hours postoperatively to all animals. The animals were supplied with rabbit pellet chow and water ad libitum. After a latency of 4 days, the tibia was lengthened 1 mm every 12 hours for 10 days, producing a total of 20 mm of distraction. The fixator was left in situ to allow the regenerate to consolidate.
Animals were randomized into 2 groups. At the end of the distraction period, the DBM group (28 animals) was injected with 100 mg of DBM at the distraction gap percutaneously (Fig. 1). DBM used in this study was in an injectable putty form (DBX DBM, Musculoskeletal Transplant Foundation, DePuy Synthes, West Chester, PA, USA). The control group (22 animals) was left without manipulation.
At the time of harvest, rabbits were killed with intravenous injection of sodium pentobarbital (150 mg/kg). Specimens were harvested from 6 animals in the DBM group and 4 animals in the control group at the third and sixth weeks of consolidation. Specimens from 2 or 3 animals were harvested at 7 additional time points (days 0, 2, 4, 7, weeks 2, 4, and 8 of consolidation) for histologic analysis. Seven rabbits were excluded during the experiment because of unintended death or technical failures before the end of lengthening, and replaced by additional animals.
During the consolidation period, plain radiography was performed every week. Under anesthesia with intramuscular ketamine (50 mg/kg) for sedation, the operated limbs were oriented in the standard anteroposterior (AP) projection and plain radiography was performed with a Siemens Multix H/UPH configuration (Siemens AG, Munich, Germany; 50 kVp, 4 mAs). We used digital luminescent cassettes and a tube to film distance of 1.1 m. A calibrated marker on the film allowed the image to be rescaled for measurements of length in millimeters. The sequential plain radiographs were compared between the DBM group and the control group. In addition to simple comparison with the naked eyes, we quantified bone density by analyzing pixels. The Image J program (National Institutes of Health, Bethesda, MD, USA) can calculate pixel values from the plain radiograph. The pixel values of the distraction gap were normalized by those of the ipsilateral tibial shaft. After normalizing the pixel values of the distraction gap, we could quantitatively compare bone density of the distraction gap between the DBM group and the control group.
In order to measure bone mineral density (BMD), we used specimens harvested at the first, third, and sixth weeks of the consolidation period. A total body dual-energy X-ray (DXA) scanner (DPX, Lunar, Madison, WI, USA) and software which was tailored for small animals (LUNAR DPX, Small Animal Software, 1.0c, Lunar) were used. The operated tibiae oriented in the AP projection were examined by the DXA scanner. Regional BMD measurements were obtained by placing rectangular (18 mm × 10 mm) regions of interest (ROI) on the scan images. For each lengthened tibia, one ROI was positioned in the distraction gap and the other ROI was positioned on the normal metaphysis proximal to the distraction gap.25) BMD data from ROI in the distraction gap was normalized by data from ROI in the normal metaphysis. Results of BMD were expressed as grams per square centimeter.
For analyzing bone morphometry, micro-computed tomography (micro-CT) was used. The distraction gap in the operated tibiae was cut and the margin of these samples was 5 mm. These samples were scanned parallel to the long axis by a micro-CT scanner (Skyscan 1076; Bruker, Kontich, Belgium). The region included nearly 1,000 images with a resolution of 2,048 × 2,048 pixels and with an isotropic voxel size of 10 µm. The system was set to 70 kV, 114 mA, and 500 ms integration time, which permitted accurate bone morphometric analysis of the distraction gap. For analyzing the structure of trabecular bone, specimens harvested at the third week of the consolidation period were examined. For analyzing the thickness of cortical bone, specimens harvested at the sixth week of the consolidation period were examined. We reconstructed 3-dimensional images from the micro-CT data to analyze the structure of trabecular bone. In order to minimize the effect of bone marrow cavities, total nine volume of interests (VOIs) adjacent to the bone marrow cavity were selected in three levels of the distraction gap. The VOI was set to have 35 × 35 × 35 voxels, and we reconstructed 3-dimensional images of these VOIs. The following microarchitecture parameters, bone volume to total volume ratio (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N), were assessed from the reconstructed 3-dimensional images. BV/TV indicates the portion of mineralized tissue, and Tb.Th, Tb.Sp, and Tb.N provide information on the thickness and amount of the trabeculae. We reconstructed binary images from the micro-CT data to analyze cortical thickness of the regenerated bone.
All harvested specimens were used for examining the histology. The specimens were bisected longitudinally. They were fixed in paraformaldehyde (4%, pH 7.4) at 24℃ for 48 hours. For decalcification, they were buffered with ethylenediaminetetraacetic acid (14.5%)/paraformaldehyde (0.5%, pH 8.0) at room temperature for 5 weeks. Simple radiography was performed for identifying decalcification. The distraction gap was resected from the operated tibia and embedded in paraffin wax. This paraffin block was cut sagittally (5 µm). We used H&E staining and safranin O and fast green staining for examining the histology.
Sequential images of plain radiography revealed that the DBM group showed more callus formation and faster regeneration than the control group (Fig. 2). At the third week of the consolidation period, callus was more abundant in the DBM group. At the sixth week of the consolidation period, the DBM group showed fusiform-like callus formation with corticalization. At the eighth week of the consolidation period, corticalization was more apparent in the DBM group and insufficiency in bone remodeling was observed in the control group (Fig. 2). Pixel values for the distraction gap area indicated that the DBM group showed higher pixel values than the control group at the early consolidation period (at 2 and 3 weeks, p < 0.05) and pixel values became similar between the two groups after the fourth week of the consolidation period (Fig. 3). In the DBM group, pixel values at the third week of the consolidation period were highest and showed a 49% increase over pixel values at the beginning of the consolidation period. In the control group, pixel values at the sixth week of the consolidation period were highest and showed a 43% increase over pixel values at the beginning of the consolidation period. The DBM group showed 46% higher pixel values than the control group at the second week of the consolidation period, and this was the largest difference. After the second week of the consolidation period, the difference in pixel values between the DBM group and the control group was diminished.
Result of BMD analysis coincided with the result of radiography analysis. The DBM group showed highest BMD score at the third week of the consolidation period and the BMD score in the DBM group decreased at the sixth week of the consolidation period. The control group showed a consistent increase in the BMD score from the first week to the sixth week of the consolidation period. At the third week of the consolidation period, the DBM group showed a 64% higher BMD score than the control group. At the sixth week of the consolidation period, the DBM group showed a 14% lower BMD score than the control group (Fig. 4).
We reconstructed 3-dimensional images from micro-CT data of the third week of the consolidation period for analyzing the trabecular bone structure. The reconstructed 3-dimensional images showed that the DBM group had more abundant trabecular bone formation than the control group at the third week of the consolidation period. At the same time, the micro-architecture parameters of the DBM group were compared with those of the control group. The DBM group showed statistically higher values of BV/TV, Tb.Sp, and Tb.N than the control group. However, Tb.Th was not statistically different between the DBM group and the control group (Table 1). The DBM group showed 14% higher BV/TV, 50% lesser Tb.Sp, and 34% more Tb.N than the control group at the third week of the consolidation period. These differences were statistically significant. The binary images, which were transformed from 2-dimensional images for analyzing the thickness of the cortex at the sixth week of the consolidation period, showed an apparent difference in cortical thickness between the DBM group and the control group (Fig. 3).
We performed histologic examination to investigate the difference in bone regeneration process between the DBM group and the control group. At the beginning of the consolidation period, we injected DBM into the distraction gap percutaneously. On the second day of the consolidation period, we observed that the woven bone architecture was distorted and inflammatory cells had infiltrated around the injected DBM. At the third week of the consolidation period, the DBM group showed more apparent remodeling and bone regeneration than the control group. We performed safranin O and fast green staining for visualizing the cartilaginous component. We observed the cartilaginous component in the DBM group, and this component was not observed in the control group. The presence of the cartilaginous component indicated that endochondral bone formation had occurred around the DBM. At the sixth week of the consolidation period, the DBM group showed a larger diameter of regenerated bone and thicker cortical bone than the control group. The injected DBM was absorbed slowly; hence, it could be observed at the sixth week of the consolidation period (Fig. 5).
We demonstrated in this study that percutaneous injection of DBM into the distraction gap during the consolidation period of DO can enhance bone regeneration via inducing endochondral bone formation.
Since Ilizarov reported the concept of DO, it has been accepted as a powerful technique for achieving bone regeneration. The principle of DO is that gradual distraction after low-energy corticotomy with stable fixation can regenerate bone. Advantages of DO include possibility of regenerating large volume of autologous bone, increased vasculogenesis, and potential for deformity correction. However, DO technique has disadvantages such as long duration of bone healing and need for large external devices leading to patient discomfort. To the best of our knowledge, there is no biomechanical study investigating when large external devices can be removed safely. But there is a consensus that the removal of large external devices cannot be permitted until at least 3 of 4 cortices are observed in two orthogonal simple radiographic views (e.g., anteroposterior and lateral).10) If we can accelerate the process of bone regeneration and bone maturation, we can reduce the duration of the use of large external devices, and it could eventually reduce patient inconvenience and complications during DO.
The ideal material for accelerating bone regeneration and bone maturation during DO should be easily accessible and efficacious, and should have low comorbidity. We focused on DBM because it has already been proved to be efficient in bone regeneration and is clinically available without any comorbidity. Hence, we attempted to test whether DBM can enhance bone formation during DO in more clinically relevant settings (normal speed of distraction and injection of DBM after the end of distraction).
In our study, the pixel value analysis and the BMD analysis showed higher bone density in the DBM group than in the control group at the third week of consolidation. The micro-architecture parameter analysis showed that the DBM group showed increased trabecular bone formation than the control group at the third week of the consolidation period. The DBM group also showed increased trabecular bone regeneration than the control group in the histological examination. These results of our study proved that the DBM group showed increased amount of regenerated bone than the control group. In addition to this result, our study also showed that the DBM group showed faster remodeling of the regenerated bone than the control group. The 2-dimentional binary image analysis showed that cortical bone in the DBM group was thicker than that in the control group at the sixth week of the consolidation period, and histologic findings showed that cortical thickness and bone diameter in the DBM group were greater than those in the control group at the eighth week of the consolidation period. The thickness of cortical bone was a type of parameter which represented bone remodeling and bone stiffness. Therefore, our study showed that DBM had effects of enhancing bone regeneration and quickening bone remodeling.
In normal DO without the use of DBM, intramembranous ossification was a predominant mode of bone regeneration after the early distraction period of DO when the endochondral ossification was a predominant mode of bone regeneration.26) During the distraction period, microcolumns were formed in front of both corticotomy sites, and a fibrous intermediate zone existed in the center of the distraction gap. After the distraction period, both sides of micro-columns were interconnected.27) In the late distraction period, preosteoblasts which were present around the micro-column differentiated into osteoblasts and mineralization of micro-columns occurred. At the end of mineralization, osteoblasts differentiated into osteocytes and these osteocytes were encased by mineralized bone.28) In this study, we found that regenerated bone in the DBM group contained cartilage tissues surrounding the injected DBM during the consolidation period, which could be interpreted as endochondral ossification in the DBM group. This difference might be due to BMP present in the injected DBM. In normal DO without the use of DBM, the BMP level was increased during the latency period, achieved its peak in the distraction period, and was decreased after the distraction period.26) But in our study, the effect of BMP may have persisted during the consolidation period, because DBM was injected at the beginning of the consolidation period.
There might be controversies on the timing of injection of DBM during DO. We thought that in case of successful bone regeneration, adjuvant treatment might not be necessary. Furthermore, we were concerned about the possibility of premature consolidation during the distraction stage if we had injected DBM at the start or middle of the distraction period. Hence, we selected the end of distraction as the timing for DBM injection. Based on our result that percutaneous DBM injection during the consolidation period could enhance bone regeneration, we thought that selective DBM injection in patients with delayed bone regeneration at the distraction site could be possible. After percutaneous injection, the regenerated bone was distorted by injection of DBM but enhancement of bone regeneration could cause early remodeling to form a more stable cortical bone at 6 weeks.
Our study had some limitations. The sample size was not large enough (6 animals in the experimental group and 4 animals in the control group) to show statistically significant differences in some parameters. We think that a further study with a more controlled and focused experiment will be able to show statistically significant results. Also, we did not perform a mechanical study to compare bone stiffness between the DBM group and the control group. Although cortical bone thickness might be a good parameter which could represent bone stiffness, a mechanical study is needed for safe application of the results of our study to a clinical situation. Furthermore, to evaluate the effect of injection procedure itself, a sham-operated group (injection procedure without DBM injection) should be included. However, by showing histologic changes around DBM after percutaneous DBM injection, we thought that we could observe the effect of DBM on enhancement of bone regeneration in the distraction gap.
DBM administration into the distraction gap at the end of the distraction period resulted in a significantly greater regenerate bone area, Tb.N, and cortical thickness in the rabbit tibial DO model. These data suggest that percutaneous DBM administration at the end of the distraction period or in the early consolidation period may stimulate regenerate bone formation and consolidation in a clinical situation with delayed bone healing during DO.
Figures and Tables
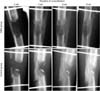 | Fig. 2At week 0, distraction gap in demineralized bone matrix (DBM) group looked more radiopaque due to injected DBM. At week 3, more exuberant bone formation was observed in DBM group. At week 6, cortical remodeling was observed in DBM group while it was scarce even at week 8 in control group. |
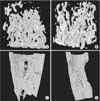 | Fig. 3The three-dimensional (3D) reconstruction image of one voxel of interest showed that demineralized bone matrix (DBM) group (A) had denser trabecular bones than control group (B) at third week of consolidation period. The 3D reconstruction image of whole regenerate in distraction gap showed that DBM group (C) had well remodeled cortices when compared with control group (D) at the sixth week of consolidation period. |
 | Fig. 4(A) Demineralized bone matrix (DBM) group showed higher pixel values than control group during early 3 weeks of consolidation period (*p < 0.05). However, during late 4 weeks of consolidation period, pixel value of DBM group was similar to that of control group. (B) There was significant difference of bone mineral density (BMD) between DBM group and control group at the 3rd week of consolidation period (*p < 0.05). BMD of DBM group was similar to that of control group at the 6th week of consolidation period. |
 | Fig. 5(A, B) Fig. 5B is a 4 times magnified picture of the black square in Fig. 5A. On the second day of consolidation period, injected demineralized bone matrix (DBM) distorted bone regenerate in distraction gap and inflammatory cells accumulated around the injected DBM (H&E). (C, D) At the second week of consolidation period, endochondral ossification around injected DBM (safranin O and fast green staining) and resorption of DBM by multi-nucleated giant cell (arrows, H&E) were observed. (E, F) At the third week of consolidation period, DBM group (E) had denser bone trabeculae than control group (F) (H&E). (G, H) At the sixth week of consolidation period, DBM group (G) showed thicker cortical bone and wider total bone diameter than control group (H) (H&E). The remnants of injected DBM were still observed at the sixth week of consolidation period (G). |
Table 1
Results of Micro-Architecture Parameters (Comparison between the DBM Group and the Control Group)

ACKNOWLEDGEMENTS
This study was supported by the Seoul National University Hospital Research Fund (03-2008-0070) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A2044067).
References
1. Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002; 17(4):435–447.

2. Catagni MA, Guerreschi F, Holman JA, Cattaneo R. Distraction osteogenesis in the treatment of stiff hypertrophic nonunions using the Ilizarov apparatus. Clin Orthop Relat Res. 1994; (301):159–163.

3. Paley D. Treatment of tibial nonunion and bone loss with the Ilizarov technique. Instr Course Lect. 1990; 39:185–197.
4. Moseley CF. Leg lengthening: a review of 30 years. Clin Orthop Relat Res. 1989; (247):38–43.
5. Korzinek K, Tepic S, Perren SM. Limb lengthening and three-dimensional deformity corrections: a retrospective clinical study. Arch Orthop Trauma Surg. 1990; 109(6):334–340.

6. Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994; (301):31–37.

7. Curran AR, Kuo KN, Lubicky JP. Simultaneous ipsilateral femoral and tibial lengthening with the Ilizarov method. J Pediatr Orthop. 1999; 19(3):386–390.

8. Noonan KJ, Leyes M, Forriol F, Canadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation: a study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998; 80(6):793–806.

9. Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990; (250):81–104.

10. Sailhan F. Bone lengthening (distraction osteogenesis): a literature review. Osteoporos Int. 2011; 22(6):2011–2015.

11. Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis: a preliminary result of three cases. Bone. 2004; 35(4):892–898.

12. Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone. 2007; 40(2):522–528.

13. Zheng LW, Cheung LK. Effect of recombinant human bone morphogenetic protein-2 on mandibular distraction at different rates in a rabbit model. Tissue Eng. 2006; 12(11):3181–3188.

14. Li G, Bouxsein ML, Luppen C, et al. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. J Orthop Res. 2002; 20(4):779–788.

15. Zakhary K, Motakis D, Hamdy RH, Campisi P, Amar Y, Lessard ML. Effect of recombinant human bone morphogenetic protein 7 on bone density during distraction osteogenesis of the rabbit mandible. J Otolaryngol. 2005; 34(6):407–414.

16. Okazaki H, Kurokawa T, Nakamura K, Matsushita T, Mamada K, Kawaguchi H. Stimulation of bone formation by recombinant fibroblast growth factor-2 in callotasis bone lengthening of rabbits. Calcif Tissue Int. 1999; 64(6):542–546.

17. Chang F, Mishima H, Ishii T, et al. Stimulation of EP4 receptor enhanced bone consolidation during distraction osteogenesis. J Orthop Res. 2007; 25(2):221–229.

18. Song HR, Oh CW, Kyung HS, et al. Injected calcium sulfate for consolidation of distraction osteogenesis in rabbit tibia. J Pediatr Orthop B. 2004; 13(3):170–175.

19. Van de Putte KA, Urist MR. Osteogenesis in the interior of intramuscular implants of decalcified bone matrix. Clin Orthop Relat Res. 1965; 43:257–270.

21. Stevenson S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res. 1998; 355 Suppl. S239–S246.

22. Zhang M, Powers RM Jr, Wolfinbarger L Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J Periodontol. 1997; 68(11):1085–1092.

23. Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder: a radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987; 69(7):984–992.

24. Hagino T, Hamada Y. Accelerating bone formation and earlier healing after using demineralized bone matrix for limb lengthening in rabbits. J Orthop Res. 1999; 17(2):232–237.

25. Little DG, Smith NC, Williams PR, et al. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003; 18(7):1300–1307.

26. Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008; 87(2):107–118.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download