Abstract
Background
We aimed to describe the clinical characteristics and outcomes of unplanned excisions of synovial sarcomas.
Methods
In total, 90 patients with synovial sarcomas in the extremities were retrospectively reviewed. Patients were divided into unplanned excision (n = 38) and planned excision (n = 52) groups. The average follow-up period was 6 years. The clinicopathological characteristics and oncologic outcomes were compared.
Results
The unplanned excision group showed longer duration of symptoms before diagnosis (p = 0.023), smaller lesion dimensions (p = 0.001), superficial location (p = 0.049), and predilection in the upper extremities (p = 0.037). Synovial sarcomas were most commonly misdiagnosed as neurogenic tumors (56%) in the upper extremities or as cystic masses (47%) in the lower extremities. Oncological outcomes, including disease-specific survival, metastasis-free survival, or local recurrence were not significantly different between the 2 groups (p = 0.159, p = 0.444, and p = 0.335, respectively). Repeated unplanned excision (p = 0.012) and delayed re-excision (p = 0.038) were significant risk factors for local recurrence in the unplanned excision group.
Synovial sarcoma is a malignant neoplasm comprising 5% to 10% of all other adult soft-tissue sarcomas (STS).1) Similar to most STS, synovial sarcoma has an aggressive and highly metastatic propensity. However, synovial sarcomas have distinct characteristics such as predilection for young adults and relatively slow growth compared with other STS. These unusual and diverse clinical features of synovial sarcomas frequently result in delayed diagnoses and unplanned excisions (UPEs).2,3)
When UPE is performed, it is widely accepted that re-excision is usually necessary to remove the possible residual tumor or to achieve adequate resection margins.4,5,6) Re-excision using wide margins that include normal surrounding tissues may cause significant morbidity and economic burden to patients.2,7) Thus, UPEs should be avoided.
Despite the relatively high incidence of UPEs of synovial sarcomas, few studies have focused on this issue.3,8,9) Identifying the characteristics of synovial sarcomas that were misdiagnosed and underwent UPEs would be beneficial in preventing an UPEs. In the present study, we aimed to assess the characteristics and outcomes of synovial sarcomas treated with UPEs.
Patients who underwent surgery for 99 histologically proven synovial sarcomas of the extremities were included in this study. Of the 99 patients, we excluded 9 patients, including 6 with less than 2 years of follow-up and 3 with insufficient medical records; thus, 90 patients were eventually examined. All data were obtained retrospectively using medical records and radiographs after approval by the relevant Institutional Review Board.
Of the 90 patients, 34 were female and 56 were male. The mean age at diagnosis was 32.7 years (range, 5 to 80 years), including 20 patients (22%) younger than 20 years. The mean follow-up period was 6 years (range, 2 to 23 years). The lower limbs including the pelvis (n = 73, 81%) were more commonly involved than the upper limbs (n = 17, 19%). For analytical purposes, patients were divided into 2 groups: the planned excision (PE) group (n = 52, 58%), who received standard treatments for synovial sarcomas with no previous unplanned procedures, or the UPE group (n = 38, 42%), who underwent surgical treatments and in whom the possibility of sarcoma was not considered initially. Thirty-three patients (87%) of the UPE group were referred to our institutions after UPEs at other hospitals.
The medical records and magnetic resonance imaging (MRI) scans of patients in the UPE group, documented at the time of initial UPEs, were reviewed to identify the initially presumed diagnoses made at referring hospitals. We regarded pain, presence of swelling, tenderness and an enlarging mass as symptoms. Histopathology specimens obtained at the time of UPEs were examined by musculoskeletal pathologists of our institution. The verified tumor characteristics included the nature of tumor contents, size, anatomical location, depth, histological subtype, and histological grade. Histological grading was performed using the Federation Nationale des Centres de Lutte Contre le Cancer grading.10) For UPE, histological grading was performed by using specimens obtained at the time of UPEs or residual tumors detected during re-excisions. Out of 90 patients, MRI scans done prior to initial surgery were available for 73 patients (81%). Every patient in the UPE group had MRI scans prior to re-excision at our institute. Tumor size was defined by the maximal dimensions reported by pathological or MRI examinations. Tumor depth was graded as superficial or deep; tumors located exclusively above the superficial fascia without invasion of the fascia were defined as superficial.11)
Treatment principles comprised wide excision with or without adjuvant chemotherapy or radiotherapy. Surgery was performed to achieve wide margins by removing the cuff of normal tissues surrounding the tumors. Limb-sparing operations were primarily performed, if possible. All patients of the UPE group underwent re-excision to achieve wide resection margins. Adjuvant chemotherapy or radiotherapy was administered postoperatively when a higher risk of recurrence was believed to exist based on the clinical information, including histological grade, tumor size, presence of metastasis, and surgical margins. However, no prospectively determined criteria were used for patient selection. Adjuvant chemotherapy and radiation therapy were administered for 18 patients (47%) and 24 patients (63%), respectively. Disease-specific survival, metastasis-free survival, or local recurrence-free survival was assessed for documenting oncological outcomes. Specific deaths due to synovial sarcomas were documented as end points for disease-specific survival. Deaths by other causes were documented as censored. Local recurrence was defined as the first recurrence of disease at the primary tumor site after resection.
Overall survival, metastasis-free survival, and local recurrence-free survival were estimated using the Kaplan-Meier estimator. Survival curves for both groups were evaluated using the log-rank testing for univariate factors and Cox's stepwise regression for multivariate factors. Relative risks were calculated using a proportional-hazards model. Comparison of the categorical variables between the PE and UPE groups was performed using the Fisher exact test and Pearson chi-square test. Statistical analyses were performed using the PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Among the clinical characteristics, the duration of symptoms before diagnosis was significantly longer in the PE group than that in the UPE group (p = 0.023) (Table 1). UPEs were more often performed for small (< 5 cm) and superficially located lesions (p = 0.001 and p = 0.049, respectively). Of the 20 upper-limb lesions, UPEs were performed for 12 (60%), compared to 26 (36%) of the 73 lesions located in the lower limb (p = 0.037). Initial metastases were detected in 13 patients (14%). The proportion of patients presenting with metastasis at diagnosis was not significantly different between the PE group (10 patients, 19%) and UPE group (3 patients, 8%; p = 0.112).
MRI scans prior to initial UPEs were available for 26 patients (68%) in the UPE group. Gadolinium enhancement was used in only 8% of cases in the UPE group compared to 96% in the PE group (p < 0.01). When the initial MRI reports of the 26 UPE patients were analyzed, a misdiagnosis pattern was observed with regard to tumor location (Table 2). In the lower limbs, almost half (8/17, 47%) of synovial sarcomas were misdiagnosed as cystic masses (popliteal cysts or meniscal cysts) around the knee (Fig. 1), whereas synovial sarcomas were most commonly misdiagnosed as neurogenic tumors (5/9, 56%) in the upper limbs (Fig. 2).
At the time of analysis, 26 patients (33%) had died of causes associated with synovial sarcoma and 2 patients had died of other causes. Five-year oncological outcomes, including disease-specific survival (64% vs. 73%, p = 0.159), metastasis-free survival (65% vs. 73%, p = 0.444), or local recurrence-free survival (77% vs. 79%, p = 0.335), were not significantly different between the PE and UPE groups, respectively (Fig. 3).
The number of UPEs prior to definitive re-excision ranged from 1 to 4 (mean, 1.36). Patients with a multiple number of UPEs (n = 8) had significantly higher rates of local recurrence than patients (n = 30) with single-excision operations (p = 0.012). The median interval between UPEs to re-excisions was 6 weeks (range, 1 to 132 weeks). Patients with intervals longer than 4 weeks (n = 19) to re-excision had significantly higher rates of local recurrence than patients with intervals shorter than 4 weeks (n = 19) to re-excision (p = 0.038). Of the 38 patients in the UPE group, residual tumors were detected in re-excised specimens in 31 patients (82%), including 29 with macroscopic residual tumors. However, the local recurrence rate did not significantly differ between the patients with residual tumors and patients without residual tumors (19% vs. 14%, p = 0.617).
Synovial sarcoma is often managed erroneously by UPEs because they are often misdiagnosed as benign masses. Synovial sarcomas are misdiagnosed because they occasionally shows atypical STS characteristics such as early onset in young adults and slow growth. There have been no reports documenting misinterpretations of imaging studies or clinical outcomes of synovial sarcomas inadequately treated with UPE. Our study compared oncological outcomes of synovial sarcomas following UPE with those treated by standard procedures.
Synovial sarcomas are frequently diagnosed late or excised inadequately because of their slow growth rates and apparently harmless symptoms.7,12) A study of the presentations of 33 synovial sarcoma patients found that only half of synovial sarcoma patients had typical STS symptoms and the mean delay until correct diagnosis was 50 weeks.7) The average duration of symptoms ranged from 2 to 4 years, and up to 20-year histories have been reported.1) Indeed, the duration of symptoms until diagnosis was significantly longer in the UPE group than in the PE group in our study. Moreover, synovial sarcomas are reportedly misdiagnosed most frequently as benign lesions by MRI, whereas only one-third of synovial sarcomas actually show benign characteristics.9,13) Synovial sarcomas present as well-defined masses with smooth, round, or gently lobulated margins and are usually localized rather than invading surrounding structures.14)
The review of MRI scans of UPE cases revealed a clear trend for anatomical location and tumor size. Our data showed that common misdiagnoses differed according to tumor location; synovial sarcomas were most commonly mistaken for neurogenic tumors in the upper limbs (56%) and cysts in the lower limbs (47%). Relatively small-sized synovial sarcomas tended to be more homogenous on MRI scans, making it difficult to diagnose them differentially from neurogenic tumors in the upper limbs.8) On the other hand, relatively large-sized synovial sarcomas were usually heterogeneous with cystic or necrotic components, making them likely to be diagnosed as cystic masses in the lower limbs.14) Contrast-enhanced MRI has been reported to be effective in differentiating a STS from a benign soft tissue tumor.8,9,14) In accordance with previous reports, most erroneous diagnoses were made using MRI scans without contrast enhancement.
The incidence of the detection of residual tumors at re-excisions was high (82%) in the current study cohort compared to reports studying other STS from 23.6% to 45%.4,5,6,15) However, the local control rates did not differ. According to these results, synovial sarcoma has tendency to show residual lesion after UPE than other kind of STS. Despite the incidence of residual tumors being 82%, the authors reported a 5-year local recurrence-free survival of 79%.
Oncological outcomes were similar between the PE and UPE groups in our study despite the potential negative effects of the complications of UPEs on patient outcome. The comparable oncological outcomes observed in both the UPE and PE groups could be attributed to the fact that known prognostic factors, such as small in tumor size and superficial in location, were more favorable in the UPE group.16,17) In the UPE group, delayed re-excisions and repeated UPEs were associated with poor local control in contrast to a previous report.4) Therefore, it is advisable for the patients to undergo re-excision as soon as possible.
We studied a relatively small patient cohort because cases of synovial sarcomas are rare. However, our report is unique because we studied the largest series of synovial sarcoma cases that had undergone UPEs, to date. Although, this study was conducted in 2 separate tertiary-referral sarcoma centers, the same principles of treatment were applied to all patients. Immediate re-excisions were performed for all the UPE patients and aggressive adjuvant treatment was recommended for all patients with residual tumors.
In conclusion, synovial sarcomas treated with UPEs had distinct characteristics, including small lesion sizes, superficial location and high risk of residual lesion. UPE was done more frequently in the patient with MRI without enhancement. Synovial sarcomas were most commonly misdiagnosed as neurogenic tumors in the upper extremities or as cystic masses in the lower extremities. Contrast-enhanced MRI is effective in differentiating a malignant mass from a benign mass in synovial sarcoma diagnosis. These findings are important for developing diagnostic and therapeutic strategies for synovial sarcoma.
Figures and Tables
Fig. 1
(A) The axial view of T1-weighted magnetic resonance imaging (MRI) scan shows a multiseptated cystic lesion in the popliteal area. (B) The T2-weighted MRI scan shows internal fluid-fluid level suggesting hemorrhagic component. (C) The mass was not enhanced on postcontrast T1-weighted MRI scan.
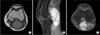
Fig. 2
(A) The coronal view of T1-weighted magnetic resonance imaging (MRI) scan shows a round lesion besides coracobrachialis muscle. (B) The axial view of postcontrast T1-weighted fat suppression MRI scan shows well-enhancing mass. (C) The T2-weighted MRI scan suggests the mass arises from the median nerve.

Fig. 3
Kaplan-Meier analyses of oncologic outcomes between the planned excision group and unplanned excision group. No statistical difference was observed between the groups: (A) disease-specific survival (p = 0.159), (B) metastasis-free survival (p = 0.335), and (C) local recurrence-free survival (p = 0.444).

Table 1
Comparison of Clinical Characteristics between Planned Excision Group and Unplanned Excision Group

Table 2
Initial Magnetic Resonance Imaging Diagnoses of Unplanned Excision Group
References
1. Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998; 2(6):401–421.
2. Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008; 90(2):203–208.
3. Trovik CS. Scanadinavian Sarcoma Group Project. Local recurrence of soft tissue sarcoma: a Scandinavian Sarcoma Group Project. Acta Orthop Scand Suppl. 2001; 72(300):1–31.
4. Han I, Kang HG, Kang SC, Choi JR, Kim HS. Does delayed reexcision affect outcome after unplanned excision for soft tissue sarcoma? Clin Orthop Relat Res. 2011; 469(3):877–883.
5. Qureshi YA, Huddy JR, Miller JD, Strauss DC, Thomas JM, Hayes AJ. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol. 2012; 19(3):871–877.
6. Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer. 2003; 97(10):2544–2553.
7. Chotel F, Unnithan A, Chandrasekar CR, Parot R, Jeys L, Grimer RJ. Variability in the presentation of synovial sarcoma in children: a plea for greater awareness. J Bone Joint Surg Br. 2008; 90(8):1090–1096.
8. Valenzuela RF, Kim EE, Seo JG, Patel S, Yasko AW. A revisit of MRI analysis for synovial sarcoma. Clin Imaging. 2000; 24(4):231–235.
9. Blacksin MF, Siegel JR, Benevenia J, Aisner SC. Synovial sarcoma: frequency of nonaggressive MR characteristics. J Comput Assist Tomogr. 1997; 21(5):785–789.
10. Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol. 2004; 22(20):4040–4050.
11. Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 6th ed. New York: Springer;2002.
12. Krieg AH, Hefti F, Speth BM, et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011; 22(2):458–467.
13. Berquist TH, Ehman RL, King BF, Hodgman CG, Ilstrup DM. Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. AJR Am J Roentgenol. 1990; 155(6):1251–1255.
14. Stacy GS, Nair L. Magnetic resonance imaging features of extremity sarcomas of uncertain differentiation. Clin Radiol. 2007; 62(10):950–958.
15. Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006; 13(1):110–117.
16. Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005; 16(3):437–444.
17. Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004; (419):155–161.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download