Abstract
Acromioclavicular (AC) and sternoclavicular (SC) septic arthritis with contiguous pyomyositis are rare, especially in immunocompetent individuals. We report a case of septic AC joint with pyomyositis of the deltoid and supraspinatus muscles and a separate case with septic SC joint with pyomysitis of the sternocleidomastoid muscle. Both patients had similar presentations of infections with Staphylococcus aureus and were successfully treated with surgical incision and drainage followed by prolonged antibiotic therapy.
Acromioclavicular (AC) and sternoclavicular (SC) septic arthritis with juxta pyomyositis is rare, with few reports of cases from temperate North America. Pyomyositis has an estimated incidence of 0.5 cases per 100,000 person-years,1) with only 8% of these in the shoulder2) and even fewer in the sternocleidomastoid (SCM) muscle.3) Risk factors for both pyomyositis and septic arthritis include diseases such as acquired immunodeficiency syndrome (AIDS), diabetes, intravenous drug abuse (IVDA), and malignancy.4,5) Prompt recognition of pyomyositis and septic arthritis is necessary for proper treatment and prevention of sepsis, chronic osteomyelitis, and even death. Herein, we report two cases, an AC joint and an SC joint infection with contiguous pyomyositis.
A 42-year-old overweight immunocompetent woman with a history of hypertension and mild psoriasis and no travel history was seen twice in the emergency department-11 days prior to admission and again 8 days prior to admission-with tachycardia, tachypnea, and left infrascapular back pain that radiated to her chest. Her white blood count was 8.52 K/µL (normal range, 3.7 to 10.4 K/µL), and chest radiograph and computed tomographic (CT) scan were negative for any bony abnormalities or cardiopulmonary pathology. The patient was given muscle relaxants and narcotic medication on both occasions and discharged home.
Five days prior to admission, the patient reported using her arms to help stand up and immediately feeling a "pop" in her shoulder with associated pain. She saw her primary care physician 2 days later for evaluation. Her upper back pain had diminished; however, her shoulder was noted to be erythematous, warm, and diffusely tender to palpation. Range of motion of the shoulder joint was decreased in abduction. A complete blood count revealed leukocytosis at 15 K/µL. The patient was prescribed cefadroxil 1 g twice daily for 10 days of treatment for presumed cellulitis of the shoulder and anterior chest.
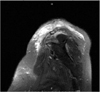
The patient again returned to her primary care physician with a fever, increased pain, and decreased range of motion in her shoulder. She had an ultrasound-guided aspiration of the left AC joint, blood cultures were taken, and she was admitted to the hospital. Her admission testing revealed a white blood cell count of 18 K/µL, a platelet count of 660 K/µL (reference range, 140 to 385 K/µL), a C-reactive protein concentration of 9.9 mg/dL (normal range, 11.8 to 15.1 mg/dL), erythrocyte sedimentation rate of 60 mm/hr (normal range, < 25 mm/hr), and blood cultures negative for growth. A CT scan of the patient's shoulder revealed no signs of osteomyelitis. A magnetic resonance imaging (MRI) scan without contrast of the left shoulder revealed increased fluid collection on short TI inversion recovery (STIR) images in the anterior deltoid and supraspinatus, as well as in the AC joint (Fig. 1). No hyperintensity on STIR images was noted in the glenohumeral joint. Cultures from the AC joint revealed a Staphylococcus aureus susceptible to methicillin, but due to a penicillin allergy, she was initially treated with intravenous (IV) vancomycin therapy and later changed, based on susceptibility testing, to 900 mg clindamycin IV 3 times a day while in the hospital.
An open incision and drainage was performed on hospital day 2. A large amount of necrotic tissue and pus was drained from the anterior deltoid and supraspinatus muscles, and a small amount from the AC joint. The clavicle was soft with cystic changes compatible with osteomyelitis, and a small fragment of the distal clavicle was excised.
Immediately after surgery, the patient felt better, had improved shoulder range of motion and decreased erythema, and was discharged on hospital day 5 on 300 mg oral clindamycin 3 times a day for 25 days to complete 5 weeks of antibiotic treatment. On her follow-up appointment 2 weeks after discharge, she was feeling better and had no further signs of infection, and at 5 months had a full range of her shoulder function without signs of recurrent infection.
Over 4 or 5 days prior to her admission to hospital, a 50-year-old woman developed increasing pain erythema, and swelling over the left SC joint. The patient's past medical history was noteworthy for a remote history of IVDA, hypothyroidism, and hypertension. Pertinent findings were pain and swelling over the left SC joint with pain in the left contiguous SCM muscle, but no definite fluctuance. Joint aspiration revealed frank pus, and gram-positive cocci were seen on a Gram stain. On admission the patient had a blood pressure of 132/78 mmHg, pulse of 105 beats per minute, respiration rate of 18 breaths per minute, and temperature of 37.4℃.
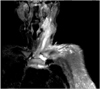
Initial laboratory results revealed no evidence of drug abuse on urine testing, a white blood cell count of 16.3 K/µL, 91% polymorphic nuclear cells, and an SC joint aspirate with 140 K/uL white blood cells. Cultures returned S. aureus susceptible to methicillin. The patient's initial antibiotics, IV vancomycin and IV piperacillin tazobactam, were therefore changed to oxacillin 2 g IV every 6 hours. On day 2 of hospitalization the tenseness and swelling over the SC joint worsened, and was incised and drained of purulent material. The next day, with increasing pain and swelling of the SCM muscle, an MR scan revealed fluid collection on STIR images in the SC joint, edema of the proximal bone, and liquefaction of the entire SCM muscle from the SC joint to the base of the skull (Fig. 2). This muscle was, therefore, incised inferiorly, drained of a large amount of purulent material, and was packed with iodoform gauze. Subsequently, the patient has responded to IV antibiotics directed against the S. aureus, first with 2 weeks of oxacillin IV followed by 4 weeks of outpatient ceftriaxone IV 2 g per day. At follow-up, her joint and left neck pain had improved, but atrophy of the SCM muscle was noted. In more than 12 month follow-up, there has been no signs of infection recurrence.
The AC and SC joints, sliding and saddle joints, respectively are non-weight-bearing and have minimal surface area. Pyomyositis and septic arthritis of either end are rare entities probably due to their unique anatomy. Although treatment modalities are similar for septic arthritis and pyomyositis, variations do exist, and rapid, accurate diagnosis can save both life and limb. Differentiating pyomyositis from septic arthritis is difficult because, as in these patient's presentations, one can mimic the other,4) so 2 distinct pathologic possibilities exist.
One possible explanation for these patients' symptoms is initial primary pyomyositis with secondary septic arthritis of the clavicular joints. Although primary pyomyositis is described as a rare disease in temperate climates, the epidemiology, clinical symptoms, course, hypothetical pathophysiology, and treatment have been well documented.1,2,3,4,5,6)
Primary pyomyositis is defined as an acute deep bacterial infection of large skeletal muscles not secondary to a contiguous skin, bone, or soft-tissue infection. Originally described in tropical climates in immunocompetent hosts, pyomyositis in immunocompromised patients living in temperate climates is becoming more prevalent. In fact, up to 75% of pyomyositis in temperate climates has been associated with underlying disease with immunodeficiency, such as diabetes mellitus, AIDS, IVDA, or malignancy.6) Infective agents vary on immune state as well as other predisposing factors. Immunocompetent patients in temperate climates are affected by S. aureus 75% of the time, often is associated with younger age, a prior traumatic event such as vigorous exercise, and underlying skin diseases.1) In contrast, immunocompromised patients are also susceptible to more unusual organisms, such as Haemophilus influenzae, Bartonella species, Pseudomonas species, enterobacteriaceae, and anaerobes.
Although the pathophysiology of pyomyositis has not yet been defined, it is believed to be due to a transient bacteremia that localizes in the muscle tissue. Muscle tissue is usually resistant to infectious agents, but in the face of an injury, the alteration of muscle structure makes it more susceptible, explained by the Latin term "locus minoris resistentiae." Increased blood supply to the damaged tissue may provide hematogenous access for the bacteria and their required nutrients.
Presentation of pyomyositis has been described in 3 stages: invasion, suppuration, and sepsis. The invasive stage begins in the first few days of onset as a dull and crampy pain.2) A patient may have a low-grade fever and mild leukocytosis. If the infection is promptly diagnosed in the invasive stage, antibiotic therapy alone may suffice, and the selection of which should be based on culture and susceptibilities and/or the epidemiology. Anti-gram-positive antibiotic therapy directed against S. aureus and beta streptococci is usually adequate for immunocompetent patients, whereas immunocompromised patients should also receive coverage for gram-negative organisms. Duration of antibiotic therapy varies from 3 to 6 weeks.
Unfortunately, 90% of patients are not treated in the invasive stage and progress in 1 to 3 weeks to the suppurative or purulent stage, as in our cases. Pus is palpable as an abscess in the muscle; however, it is typically firm, not fluctuant as was found in these cases. A patient experiences increased edema, pain, and a fever. Laboratory test results demonstrate an elevated erythrocyte sedimentation rate and leukocytosis.2) Treatment at this stage includes drainage of the abscess in addition to antibiotic therapy. Untreated pyomyositis will progress to the septic or toxic stage and possible death. Mortality ranges from 1% to 10%.
The picture of primary pyomyositis fits our first patient's presentation, which began with a vague crampy pain and progressed to abscess formation in the supraspinatus and anterior deltoid fitting the time-frame of the documented stages of primary pyomyositis. She was affected by the most common pathogen that causes pyomyositis, methicillin-susceptible S. aureus (MSSA),5) and had an underlying skin disorder, which has been associated with MSSA pyomyositis.1) However, to our knowledge, only 1 case of pyomyositis leading to septic arthritis of the SC joint has been reported, and none of the AC joint.
Alternatively, both patients may have had septic arthritis and secondary juxta pyomyositis. This seems more likely in the second case who had a remote history of IVDA. Secondary pyomyositis has been reported less often than primary pyomyositis and has been associated with non-S. aureus infections. Although primary and secondary pyomyositis present similarly, less than 20% of patients with secondary pyomyositis recover with antibiotic therapy alone.6)
To our knowledge, only 25 cases of septic arthritis of the AC joint have been recorded.7,8,9) While not as rare, SC joint septic arthritis has been reported, to be mistaken for pyomyositis.4) The epidemiology of septic arthritis is similar to that of pyomyositis. Risk factors include IVDA, local glucocorticoid injection, diabetes, AIDS, and undergoing chemotherapy for myeloma. In review of AC joint septic arthritis, patients range in age from 17 to 79 years, with an average age of 54 years. Unlike our cases, men are affected twice as often as women.
Diagnosis of sepsis involving either clavicular joints is made by aspiration, with or without ultrasound guidance. MRI is also useful in assessing septic arthritis and helps greatly, as in our cases, with assessment of surrounding muscle for suppuration and contiguous osteomyelitis. Overall, S. aureus is responsible for the arthritis in more than 50% of cases. Other pathogens include various streptococcal species, enterobacteriaceae, atypical mycobacterium, Haemophilus parainfluenzae, Candida parapsilosis, Cryptococcus, and Ochribactrum anthropi. Septicemia occurs in approximately half of patients. Complications include osteomyelitis, infective endocarditis, and death. Septic arthritis of the AC joint has also been associated with abscess formation between the surrounding muscle. Treatment options include IV antibiotics and surgical debridement with or without distal clavicle excision.7,8,9,10)
The exact pathology of our patients' septic arthritis of the AC and SC joints along with contiguous pyomyositis is unclear, and their clinical manifestations were, on review of the literature, unusual. These women had not traveled to the tropics and were not immunocompromised. Only the second patient had a remote past history of IVDA. The concomitant presence of 2 rare entities made determining their primary causation difficult; however, prolonged antibiotic therapy for septic arthritis is similar to that for pyomyositis, and treatment with surgical drainage and debridement, eradicated their infections.
Figures and Tables
References
1. Block AA, Marshall C, Ratcliffe A, Athan E. Staphylococcal pyomyositis in a temperate region: epidemiology and modern management. Med J Aust. 2008; 189(6):323–325.
2. Bickels J, Ben-Sira L, Kessler A, Wientroub S. Primary pyomyositis. J Bone Joint Surg Am. 2002; 84(12):2277–2286.
3. Park A, Lehnerdt G, Lautermann J. Myositis of the sternocleidomastoid muscle as a result of arthritis of the sternoclavicular joint. Laryngorhinootologie. 2007; 86(2):124–127.
4. Andrew JG, Czyz WM. Pyomyositis presenting as septic arthritis: a report of 2 cases. Acta Orthop Scand. 1988; 59(5):587–588.
5. Burdette SD, Watkins RR, Wong KK, Mathew SD, Martin DJ, Markert RJ. Staphylococcus aureus pyomyositis compared with non-Staphylococcus aureus pyomyositis. J Infect. 2012; 64(5):507–512.
6. Sharma U, Schwan WR, Agger WA. Escherichia coli pyomyositis in an immunocompromised host. WMJ. 2011; 110(4):182–184.
7. Sokolowski MJ, Koh JL. Pyomyositis of the shoulder girdle. Orthopedics. 2006; 29(11):1030–1032.
8. Bossert M, Prati C, Bertolini E, Toussirot E, Wendling D. Septic arthritis of the acromioclavicular joint. Joint Bone Spine. 2010; 77(5):466–469.
9. Lim KB, Kwak YG, Kim YS, Park KR. Shoulder joint infectious arthritis and acromioclavicular joint osteomyelitis due to Candida. Ann Rehabil Med. 2012; 36(4):573–577.
10. Chirag AS, Ropiak CR, Bosco Iii JA, Egol KA. Septic arthritis of the acromioclavicular joint: a report of four cases. Bull NYU Hosp Jt Dis. 2007; 65(4):308–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download