Abstract
Background
The authors conducted the present study to identify clinical and radiological prognostic factors in infants and neonates with septic arthritis of the hip.
Methods
The authors retrospectively reviewed the records of 31 patients with septic arthritis of the hip. All of the patients were younger than 18 months old. Follow-up periods ranged from 5 to 17 years. The following potential variables for predicting the prognosis were included in the assessment: gender, age, underlying diseases, duration of symptoms, changes of hip joint in X-ray, concomitant osteomyelitis, elevation of erythrocyte sedimentation rate and C-reactive protein, sepsis, pus drainage, synovial fluid culture, and infecting organisms. Clinical and radiological prognoses were analyzed at the final follow-up.
Results
Univariate analysis demonstrated that radiological prognoses were poorer in patients who had underlying diseases, a longer duration of symptoms, and pus drainage. However, on multivariate analysis, only the variable-duration of symptoms-was found to be statistically related with a poor radiological prognosis.
Septic arthritis remains an important infection, and is associated with significant mortality and morbidity.1,2,3) It is well established that the prognosis of septic arthritis in children is associated with site, age, treatment delay, and the organism responsible.4)
The hip is known to be the most frequently involved site and has the poorest outcomes of all joints.5,6) This is due to the deep location and aspiration difficulties, and results in complications of osteomyelitis of the neck of the femur in some cases. Many studies have reported that neonates and infants are more likely to have a poor prognosis than children. Particularly in neonates and infants, its diagnosis may be difficult to establish because the physical signs are frequently minimal, laboratory findings are often normal, and initial roentgenographic appearance is often unhelpful or difficult to interpret, because joint structures are primarily cartilaginous, and therefore, radiolucent.7) Accordingly, a high level of suspicion is required to make an early diagnosis.8)
The prognostic factors of septic arthritis of the hip in infants are less frequently studied than that of the children, and the reports are outdated.9) Furthermore, to our knowledge, no study has performed a multivariate analysis for the prognostic factors of septic arthritis of the hip in infancy. We speculated that the prognostic factors of septic arthritis of the hip in infancy might differ from those previously described in children because of the recent development of diagnostic tools, antibiotics, and other forms of supportive care. Therefore, we conducted the present study to identify clinical and radiological prognostic factors in infants and neonates with septic arthritis of the hip.
The Institutional Review Board of Samsung Medical Center reviewed and approved this study. After informed consent was obtained, we retrospectively reviewed the medical records and radiographs of all patients treated surgically for septic arthritis of the hip from January 1995 to December 2007. Only patients younger than 18 months old at the onset of septic arthritis were included. All the patients were followed-up for at least 5 years (range, 5 to 17 years; mean, 74.4 months). Open drainage and irrigation under general anesthesia were promptly performed after diagnosing septic arthritis of the hip in all patients. Affected hip joints were thoroughly debrided and copiously irrigated, and immobilized with a continuous suction drain in a functional position for about 2 weeks. Intravenous empirical antibiotics were used routinely and bacteriological cultures and sensitivity tests were performed. Changes were noted in the results.
After surgery, a diagnosis of septic arthritis was explicitly assigned when a patient had a positive culture finding of joint fluid and a white blood-cell count in the joint fluid of at least 50,000 cells/mm3 (50.0 × 109 cells/L). If no growth was observed during culture, we presumed a diagnosis of septic arthritis when 2 of the major and 5 of the minor clinical criteria described by Morrey et al.10) were satisfied (Table 1) and the diagnosis was supported by ultrasonographic and magnetic resonance imaging findings which showed joint distension with an increased amount of debris. We excluded the patients who had osteomyelitis alone and did not have a pathologic finding of septic arthritis in a tissue biopsy of the joint cavity. Also, cases of Mycobacterium tuberculosis arthritis of the hip were excluded.
Thirty-one patients met the study criteria. The following data were assessed for all patients included in the study: gender, age, underlying diseases including prematurity, duration of symptoms before operation, changes of hip joint in preoperative X-ray, concomitant osteomyelitis of the proximal femur, elevation of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), development of sepsis, intraoperative pus drainage, synovial fluid culture, and infecting organisms.
Of the 31 patients, 17 patients (54.8%) were male and 14 patients (45.2%) were female. Patients' ages ranged from 10 days to 18 months (mean, 3 months). Thirteen patients (41.9%) were neonates (≤ 1 month) and 18 (58.1%) were infants (from 1 month to 18 months). Seven patients (22.6%) had at least one underlying disease. The distribution of underlying diseases is summarized in Table 2. On average, surgery was conducted 4.32 days (range, 1 to 15 days) after the onset of symptoms.
Changes of the hip joint in the preoperative X-rays were observed in 12 patients (38.7%) and these changes were either single or multiple: joint space widening in seven, asymmetric soft tissue swelling in six, radiolucent lesion of proximal femur in four, and joint dislocation/subluxation in two. Four patients (12.9%) had concomitant osteomyelitis of the proximal femur. Osteomyelitis was diagnosed by the results of medullary culture and biopsy after the operation and supported by the preoperative magnetic resonance imaging (MRI) or the serial follow-up of plain radiographs, which showed the periosteal reaction, bony erosion, and an intramedullary osteolytic lesion.
At admission, ESR was elevated (> 22 mm/hr) in 26 (83.9%) and CRP (> 0.3 mg/dL) in 28 (90.3%). Sepsis was diagnosed based on core temperature, heart rate, respiratory rate, leukocyte count, and presence of infection.11) Thirteen patients (41.9%) had concomitant sepsis. Intraoperative pus drainage was observed in 23 patients (74.2%).
Joint fluid cultures were positive in 12 (38.7%): Staphylococcus aureus in six with methicillin resistant S. aureus (MRSA) in two, methicillin-resistant coagulase-negative Staphylococcus, β-hemolytic Streptococcus, Neisseria meningitidis, and Escherichia coli were cultured in one patient each, and Haemophilus influenzae in two. No patient was found to have multiple organisms in the hip joint. For statistical analysis, we categorized the infecting organisms into S. aureus, other, and none.
Clinical and radiological outcomes were analyzed at the final follow-up. The final results were each classified into 4 grades (excellent, good, fair, poor) clinically using the scale put forth by Merle D'Aubigne et al.12) (Table 3) and radiologically by the grading system of Bennett and Namnyak9) (Table 4). For statistical analysis, patients with excellent or good results were grouped as satisfactory, and patients with poor or fair results were grouped as unsatisfactory.
We calculated overall summary statistics in terms of means and standard deviations for continuous variables and frequencies and percentages for categorical variables. After the descriptive analysis, we performed univariate comparisons for the independent associations between variables and the prognosis of septic arthritis. All variables were tested for normality using the Kolmogorov-Smirnov test. The intergroup differences were determined by use of either the Student t-test or Mann-Whitney U-test for continuous variables depending on the result of normality test and the chi-square or Fisher exact test for categorical variables. Thereafter, we included all the variables in the multivariate models and performed regression analysis in a stepwise manner to identify the factors influencing the prognosis of septic arthritis. All statistical analyses were performed with the use of two-tailed tests, and significance was considered to be indicated by a p-value of < 0.05. The statistical software MedCalc ver. 11.6 (MedCalc Software, Ostend, Belgium) and R ver. 2.12 (GNU General Public License; Comprehensive R Archive Network, Boston, MA, USA) were used for all statistical analyses.
The clinical results showed 26 patients (83.9%) in the satisfactory group, with 14 excellent and 12 good. The other 5 patients (16.1%) were grouped as unsatisfactory with 3 fair and 2 poor. Univariate analysis demonstrated that no parameter was significantly associated with a worse clinical prognosis (Table 5).
Radiologically, 16 patients (51.6%) were grouped as satisfactory with 10 excellent and 6 good. The other 15 patients (48.4%) were grouped as unsatisfactory with 10 fair and 5 poor (Table 4). The underlying diseases, including prematurity, the duration of symptoms before operation, and intraoperative pus drainage were significantly associated with a worse radiologic prognosis. The radiologic results were worse when the patients had the underlying diseases (p = 0.037), when the operation was delayed (p = 0.009), or when the pus was drained during the operation (p = 0.047). Among the 7 patients with underlying diseases, only 1 patient was classified into the satisfactory group, and among the 23 with intraoperative pus drainage, only 9 were satisfactory (Tables 2 and 6). Because the duration of symptoms before operation does not follow the normal distribution statistically, we calculated the median value. It was 2.5 days (95% confidence interval, 1.00 to 4.36) in the satisfactory group and 5.0 days (95% confidence interval, 3.00 to 8.47) in the unsatisfactory group (Fig. 1). The other variables were not found to affect to the radiological prognosis significantly (Table 6).
However, the stepwise multivariate logistic regression analysis showed only one variable-the duration of symptoms before operation-to be statistically related with the radiological poor prognosis. The model was checked for goodness of fit with the Hosmer and Lemeshow test, which ensured that it was well specified and fit the data. The regression equation was as follows: prob (unsatisfactory radiological prognosis) = exp(-2.3754 + 0.5038 × duration of symptoms before operation) / [1 + exp(-2.3754 + 0.5038 × duration of symptoms before operation)] (Fig. 2).
The present study was designed to identify clinical and radiological prognostic factors of septic arthritis of the hip only in neonates and infants. Several studies have suggested prognostic factors of septic arthritis in childhood, and the putative indicators of poor prognosis include: a young age, treatment delay, septic arthritis associated with osteomyelitis, and the infecting organism.4,7,13)
In particular, septic arthritis of hip can lead to serious musculoskeletal sequelae, which include: premature closure of the triradiate cartilage, acetabular dysplasia, limb-length discrepancy, premature or asymmetrical closure of the proximal femoral physis, subluxation, dislocation, necrosis of the cartilage, ischemic necrosis of the femoral head, pseudoarthrosis of the femoral neck, and complete destruction of the femoral head and neck.2,14,15) In the present study, 5 patients had poor radiologic results and operative correction was performed in all five for the following reasons: leg length discrepancy, pathologic hip dislocation, a hip joint surface irregularity, and coxa magna (Fig. 3).
Usually, patient age is identified as an important poor prognostic factor. Septic arthritis in infancy often presents with atypical symptoms, and moreover in neonates, clinical findings can be minimal or absent,3,16) leading to a delayed diagnosis. Another reason for poor prognosis in infancy is that infants are vulnerable to osteomyelitis. In the present study, we included only infants younger than 18 months old and the analysis revealed that both the neonate and infant groups (by age) did not have a significant difference in prognosis (p = 1.00 in clinical results, p = 0.47 in radiological results).
In a series of 33 cases with acute septic arthritis in children, Chen et al.5) found that the two factors most associated with poor results were a treatment delay of more than 5 days and the presence of osteomyelitis of the proximal femur. Early diagnosis and therapy has been reported to be the most important course of action for achieving a favorable outcome in cases of septic arthritis.3,16,17) The average delay between diagnosis and arthrotomy and irrigation was 4.32 days in this study, and multivariate analysis revealed that the duration of symptoms before undergoing the operation significantly affected the prognosis. According to our logistic regression curve, when the operation was delayed by a greater number of days, the probability of an unsatisfactory radiological outcome gradually increased. If the duration of symptoms before the operation is about 5 days, then the probability of unsatisfactory radiological prognosis was 50% (Fig. 2).
Osteomyelitis of the proximal femoral metaphysis may spread directly to the epiphysis through open transphyseal vessels in those aged less than 18 months,2) and conversely, if effective treatment is delayed, the septic arthritis spreads into the bone marrow through the metaphyseal vessels because the development of cartilaginous materials of the physis is immature in infancy. It was previously reported that joint infections with osteomyelitis have a poorer prognosis.2,5,7,13) In the present study, 4 patients had concomitant osteomyelitis. However, in contrast to the previous studies, according to our analysis, concomitant osteomyelitis was not the indicative of poor prognosis. We speculated that this result might be associated with the development of diagnostic tools, such as ultrasonography and MRI.
Only 12 of our patients (38.7%) had positive growth on culture which might be influenced by the antibiotics administered in previous hospitals. Nineteen patients (61.3%) had been treated in another hospital in our study. In terms of infecting organisms, S. aureus has been well described as the most destructive and common pathogen, but H. influenzae and Pneumococcus have been found to be pathogens less likely to produce irreversible damage. The destructive nature of S. aureus may be associated with its virulence and the associated cell-mediated immunological reaction to bacterial exoprotein.18) According to our analysis, although S. aureus was the most common pathogen in accordance with the previous reports, it was not associated with a poor prognosis. This may be due to the recent development of specific antibiotics and the early application of antibiotics targeted to S. aureus. We always used the first generation of cephalosporin for empirical antibiotics. Recently, Yamagishi et al.19) reported that MRSA was the most common septic arthritis pathogen among healthcare-associated and community-acquired infections, and suggested that empiric treatment should include first-line antibiotics against MRSA in Japan. However, only 2 of our patients had a MRSA infection.
Underlying diseases, for example, respiratory distress syndrome and congenital anomalies, have been recognized as a risk factor, but not a prognostic factor, of septic arthritis in childhood.20) All 7 patients with underlying diseases had at least more than one cardiopulmonary or gastrointestinal anomaly in our series, and it was not found to be a poor prognostic factor in multivariate logistic regression analysis.
We assumed that the pus drainage during the operation might reflect the severity of infection, so we analyzed it as a potential poor prognostic factor. The pus was already established in 23 of the 31 cases during operation. However, because the formation of pus would be affected by a delay in operation, our multivariate analysis revealed that the pus was not a poor prognostic factor, while a delay of operation was a poor prognostic factor.
The present study has limitations that should be noted. First, our findings may not be generalizable due to the low incidence of septic arthritis of the hip combined with inadequate patient numbers. The sample size could not be determined a priori due to the retrospective nature of the study. This limits the power of the study and increases the risk of a type II error in presuming some factors are not associated with septic arthritis of the hip: that is, some of these factors might be associated with septic arthritis of the hip with a greater numbers of patients. However, a retrospective case study is often the most feasible method for prognostic factor identification and quantification in the surgical literature, because randomized controlled trials are mostly not possible.
In summary, because of the development of antibiotics and an increasing understanding of the nature of the disease, the prognostic factors of septic arthritis of the hip might have changed. In the present study, no variables except the duration of symptoms before the operation were associated with poor prognosis. It was a poor prognostic factor not clinically, but radiologically. However, it is reasonable that the radiological structural changes could result the pain or loss of motion in the long term, clinically. So we would like to emphasize that, although infants with poor general conditions and the radiological changes have already been established, a favorable prognosis might be expected by prompt surgical drainage and appropriate antibiotics.
Figures and Tables
Fig. 1
Box plots of symptom duration before operation between groups. The arrow denotes the average days of each group. (A) Satisfactory and unsatisfactory groups. (B) Radiological grade by Bennett and Namnyak9): excellent, good, fair, and poor.

Fig. 2
Logistic regression analysis showing the relations between duration of symptoms before the operation and radiological prognosis. The line is the curve fit from logistic regression, and represents the probability of an unsatisfactory radiological prognosis with the duration of symptoms before the operation. If the duration of symptoms before the operation is 2.53 days, the patient has an unsatisfactory radiological prognosis in 25% of cases; 4.72 days, in 50% of cases; 6.9 days, in 75% of cases; and 13.84 days, in 99% of cases.

Fig. 3
A ten-day-old boy born on the 28th week of gestation and weighing 650 g was treated for septic arthritis of the right hip by prompt surgical drainage and antibiotics 5 days after symptoms developed. He also had congenital heart disease and respiratory distress syndrome. (A) Preoperative plain radiograph of the hip joint showed soft tissue swelling (arrows). (B) Radiograph of pathologic dislocation of the femoral head at age 3 years (arrows). (C) He underwent open reduction, varizational and derotational osteotomy of the proximal femur, and trochanteric arthroplasty (arrows). (D) At age 10 years, flexion of the right hip was limited to 90°, and the clinical result was fair and radiological grade was poor.
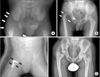
Table 1
Morrey's Diagnostic Criteria for Septic Arthritis10)

Table 2
Distribution of Underlying Diseases and the Prognoses

Table 3
Merle D'Aubigne's Clinical Grade12)

Table 4
Bennett's Radiological Grade and Number of Hips9)
Table 5
Results of Univariate Analysis of the Possible Variables for Predicting the Clinical Prognosis

Table 6
Results of Univariate Analysis of the Possible Variables for Predicting the Radiological Prognosis

ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI13C1398).
References
1. Belthur MV, Palazzi DL, Miller JA, Phillips WA, Weinberg J. A clinical analysis of shoulder and hip joint infections in children. J Pediatr Orthop. 2009; 29(7):828–833.
2. Choi IH, Pizzutillo PD, Bowen JR, Dragann R, Malhis T. Sequelae and reconstruction after septic arthritis of the hip in infants. J Bone Joint Surg Am. 1990; 72(8):1150–1165.
3. Kabak S, Halici M, Akcakus M, Cetin N, Narin N. Septic arthritis in patients followed-up in neonatal intensive care unit. Pediatr Int. 2002; 44(6):652–657.
4. Kang SN, Sanghera T, Mangwani J, Paterson JM, Ramachandran M. The management of septic arthritis in children: systematic review of the English language literature. J Bone Joint Surg Br. 2009; 91(9):1127–1133.
5. Chen CE, Ko JY, Li CC, Wang CJ. Acute septic arthritis of the hip in children. Arch Orthop Trauma Surg. 2001; 121(9):521–526.
6. Nunn TR, Cheung WY, Rollinson PD. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg Br. 2007; 89(1):100–106.
7. Shaw BA, Kasser JR. Acute septic arthritis in infancy and childhood. Clin Orthop Relat Res. 1990; (257):212–225.
8. Klein DM, Barbera C, Gray ST, Spero CR, Perrier G, Teicher JL. Sensitivity of objective parameters in the diagnosis of pediatric septic hips. Clin Orthop Relat Res. 1997; (338):153–159.
9. Bennett OM, Namnyak SS. Acute septic arthritis of the hip joint in infancy and childhood. Clin Orthop Relat Res. 1992; (281):123–132.
10. Morrey BF, Bianco AJ Jr, Rhodes KH. Septic arthritis in children. Orthop Clin North Am. 1975; 6(4):923–934.
11. Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6(1):2–8.
12. Merle D'Aubigne R. Numerical classificaion of the function of the hip: 1970. Rev Chir Orthop Reparatrice Appar Mot. 1990; (76):371–374.
13. Caksen H, Ozturk MK, Uzum K, Yuksel S, Ustunbas HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000; 42(5):534–540.
14. Howard JB, Highgenboten CL, Nelson JD. Residual effects of septic arthritis in infancy and childhood. JAMA. 1976; 236(8):932–935.
15. Morrey BF, Bianco AJ, Rhodes KH. Suppurative arthritis of the hip in children. J Bone Joint Surg Am. 1976; 58(3):388–392.
16. Obletz BE. Acute suppurative arthritis of the hip in the neonatal period. J Bone Joint Surg Am. 1960; 42(1):23–30.
17. Al Saadi MM, Al Zamil FA, Bokhary NA, Al Shamsan LA, Al Alola SA, Al Eissa YS. Acute septic arthritis in children. Pediatr Int. 2009; 51(3):377–380.
18. Wang CL, Wang SM, Yang YJ, Tsai CH, Liu CC. Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J Microbiol Immunol Infect. 2003; 36(1):41–46.
19. Yamagishi Y, Togawa M, Shiomi M. Septic arthritis and acute hematogenous osteomyelitis in childhood at a tertiary hospital in Japan. Pediatr Int. 2009; 51(3):371–376.
20. Young TP, Maas L, Thorp AW, Brown L. Etiology of septic arthritis in children: an update for the new millennium. Am J Emerg Med. 2011; 29(8):899–902.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download