Abstract
Autosomal dominant osteopetrosis (ADO) is a sclerotic bone disorder due to failure of osteoclasts. ADO poses difficulties during arthroplasty because of the increased chance for iatrogenic fractures due to sclerotic bone. ADO is divided into two types based on radiological findings, fracture risk, and osteoclast activity. These differences suggest less brittle bone in patients with ADO I compared to that of patients with ADO II, which suggests a smaller chance of preoperative fractures during cementless arthroplasty in ADO I compared with that in ADO II. A case of cementless total knee arthroplasty in a patient with ADO I is presented. Total hip arthroplasty was performed during follow-up, and known major problems related to ADO II were experienced. Therefore, the differences between ADO I and ADO II may not be clinically relevant for an iatrogenic fracture during arthroplasty in patients with ADO.
Autosomal dominant osteopetrosis (ADO) is a rare bone sclerosing disorder first described by Albers-Schönberg in 1904. The general skeletal sclerosis is suggested to be due to failure of osteoclasts to resorb bone. Two types of ADO have been identified based on radiological and biochemical findings, fracture risk, gene mutation, and osteoclast activity in vitro.1,2,3,4,5) These differences between ADO I and ADO II suggest different disease etiologies, and reclassification of ADO I as a high bone mass disorder has been proposed.5)
Arthroplasty for osteoarthritis in patients with ADO is challenging. Difficulties encountered include iatrogenic fracture during implant placement, obliteration of the medullary canal by cortical bone, increased risk for osteomyelitis, overheating and breakage of drills and saws, and longer surgical time. Nevertheless, successful cemented total knee arthroplasty (TKA) in patients with ADO has been reported.6,7) Cementless TKA was designed to obtain longer component fixation in younger patients with osteoarthritis. Therefore, cementless TKA could be considered in patients with ADO and may be related to early onset osteoarthritis.
A normal fracture risk and the ability of osteoclasts to resorb bone in patients with ADO I compared to those of ADO II suggest a smaller chance of preoperative periprosthetic fractures during cementless TKA. We present a case of cementless TKA in a patient with ADO I-related osteoarthritis of the knee and compare this with available literature on ADO.
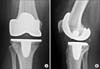
A 41-year-old female with Down syndrome and ADO I presented with a painful right knee and abnormal gait. She had no fracture history. Her only sister was also diagnosed with ADO I. On physical examination, there was effusion of the knee joint, with a correctable valgus deformity of 15° and flexion of 120°. Knee radiographs showed narrowing of the lateral and patellofemoral joint, osteophytes, loose bodies, and a dense bone structure (Fig. 1). Dual energy X-ray absorptiometry showed a bone mass density of 2.0 g/cm2 with a T-score of 6.8 for the lumbar vertebrae and a T-score of 7.5 for the right proximal femur. Lumbar spine radiographs showed no "rugger jersey spine," and pelvic radiographs showed no endobones. Skull radiographs and blood serum analysis for calcium, phosphate, acid phosphatase, and alkaline phosphatase were not performed. TKA was performed with a cementless mobile-bearing rotating platform total knee prosthesis. After extramedullary alignment, multiple saws were used to make the femoral and tibial cuts. The cementless prosthesis was extremely gently impacted. Nevertheless, a fissured fracture in the tibia occurred but was stable. Postoperative radiographs showed that the fissured fracture was distal to the cone of the tibial component (Fig. 2). Partial weight-bearing was allowed for 6 weeks after which the fissured fracture healed. Because of limited flexion to 90° at 3 months after surgery, the knee was mobilized under anesthesia. The patient was pain free, and knee flexion was 110° at 1 year after surgery. One-year postoperative radiographs showed no signs of the earlier fissured fracture (Fig. 3).
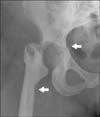
The patient presented with progressive pain and functional loss of the right hip 3 years later, which was 4 months after closed reduction of an anterior dislocation of the right hip. Hip flexion was limited to 80° with only 10° of rotation. Radiographs of the pelvis showed a dense bone structure, narrowing of the joint space, and slight dysplastic features of both hip joints. She was scheduled for a cementless total hip arthroplasty (THA). A craniocaudal acetabular fracture arose during surgery while preparing the acetabulum for the prosthesis. We prepared the femur while awaiting the osteosynthetic material. Entrance to the medullary canal was extremely difficult, and a false route occurred on the lateral side of the femoral canal. Considering both problems, we saw no possibility of achieving a stable THA and decided to conduct a resection arthroplasty (Fig. 4). She is without pain 1 year after surgery, and mainly uses a wheelchair. She is able to walk a few steps behind a walking aid. This situation is accepted by the patient and her caretaker. The patient and her caretaker were informed and agreed that data concerning the case could be submitted for publication.
This is the first English medical literature case report of cementless TKA in a patient with ADO, and particularly ADO I. Other case reports described cemented TKA in not-further-specified cases of ADO.6,7) Several distinct differences between ADO I and ADO II should be mentioned. ADO II shows end-plate thickening of the vertebrae radiologically, which is also known as "rugger-jersey spine," and endobones in the pelvis, but the calvaria are unaffected.1) ADO I almost always shows pronounced sclerosis of the skull and thickening of the cranial vault, but the spine is almost always normal.1) Serum phosphate and serum acid phosphatase levels, but not serum calcium or serum alkaline phosphatase levels, are higher in patients with ADO II compared with those with ADO I.1) Serum acid phosphatase is markedly increased outside the normal range only in patients with ADO II.1) Furthermore, an increased fracture risk is reported in patients with ADO II, compared to a normal fracture risk in those with ADO I.2) A LRP-5 gene mutation has been identified in patients with ADO I, whereas almost all cases of ADO II have been associated with a ClCN-7 gene mutation.5) In vitro ADO I osteoclasts show normal ability to resorb bone, whereas ADO II osteoclasts have impaired bone resorption.3,4) Some studies suggest that the petrified bone in patients with ADO I, is not due to failure of osteoclasts to resorb bone, but due to a gain-of-function mutation in the LRP-5 gene, which results in high bone mass; therefore, ADO I should not be considered a form of osteopetrosis.5) These differences might suggest biomechanical differences, which could have implications for iatrogenic fractures during arthroplasty. Cementless TKA is feasible with minor complications, but osteotomies of the tibia and the femur are difficult because of high bone density, and extramedullary alignment was used in our case, as described earlier in ADO-related TKA.6,7)
In our case, skull radiographs and blood serum analysis for phosphate and acid phosphatase were not performed. Elevated serum acid phosphatase is associated with ADO II and could have contradicted our radiological and hereditary findings. Serum acid phosphatase is, unlike serum alkaline phosphatase, not a standard blood test in our hospital and was not performed. The diagnosis of ADO I in this case was clear, based on diffuse osteosclerosis in the absence of a "rugger-jersey spine" and endobones in the pelvis as well as her sister who was diagnosed with ADO I.1)
Osteopetrosis has been suggested to be related to early onset osteoarthritis. Articular cartilage is a relatively compliant material and depends on the mechanical properties of its bony subchondral bed. Increased density of the subchondral bone, as in osteopetrosis, can have a profound effect on the initiation and progression of cartilage damage. Sixteen of 37 patients with ADO have been diagnosed with osteoarthritis. In 14 case reports, 21 patients with ADO had a mean age of 46 years (range, 16 to 79 years), which supports the suggestion of early onset osteoarthritis. These findings concur with the age of the patient in our case.
The tibial stem of the total knee prosthesis is press-fit during cementless TKA. A press-fit tibial component results in bone impaction around the tibial stem. However, impacting sclerotic bone can be difficult because of the high bone density. This requires more energy to get the tibial component seated and can result in a tibial fracture. In our case, cementless TKA was complicated with a fissured fracture of the tibia, which radiologically healed within 6 weeks. Another case of osteopetrosis with a femoral fracture healed with histologically more or less a normal fracture callus after 10 days but showed unorganized tissue and only small areas of lamellar bone 1 year later.8) This poor bone remodeling is likely due to the inability of osteoclasts to resorb bone in patients with ADO II. ADO I osteoclasts can resorb bone in vitro but have a decreased resorption capacity in vivo.3) This observation suggests improved bone remodeling in patients with ADO I compared to those with ADO II.
The biggest arthroplasty problems in patients with osteopetrosis are iatrogenic fractures during implant placement, obliteration of the femoral medullary canal by cortical bone, and increased risk of osteomyelitis. In our case, an iatrogenic fissured fracture occurred during placement of the tibial component with minor consequences. However, a craniocaudal fracture of the acetabulum during THA and a false route in the proximal femur due to an obliterated femoral medullary canal arose with major consequences. To reduce the risk of an iatrogenic fracture of the acetabulum, bipolar hip arthroplasty might be considered and has been described with good results at a 2-year follow-up.9) To overcome the problem of entering the medullary canal, computer-assisted fluoroscopic navigation, use of customized femoral stems, and a computer-based guiding device for preparing a cavity within the proximal femur have been described. Access to the medullary canal is obligatory during THA; therefore, THA is more demanding than TKA in patients with ADO, and a higher risk of iatrogenic fractures during THA should be anticipated.
In conclusion, we showed that cementless TKA in a young patient with osteoarthritis and a high bone mass disorder is feasible with relatively minor complications. We suggest that the difference between ADO I and II might be relevant when placing press-fit tibia stems during TKA with regard to periprosthetic fractures. However, we experienced known major problems related to ADO II during THA. Therefore, the suggested difference between ADO I and ADO II might not be clinically relevant during arthroplasty. The low prevalence of ADO and the possible arthroplasty-related major complications make referral to an experienced arthroplasty surgeon advisable.
Figures and Tables
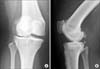 | Fig. 1Preoperative radiographs of the right knee. (A) Anteroposterior view showing dense bone, narrowing of the lateral joint space, osteophytes in the lateral and medial plateau, and valgus alignment. (B) Lateral view showing dense bone, osteophytes at the patellofemoral joint and possible loose bodies. |
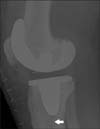 | Fig. 2Postoperative radiograph of the right knee. Lateral view shows the cementless mobile-bearing total knee prosthesis and dense bone 1 day after surgery. Arrow points to the fissured fracture that was not visible on the anteroposterior view. |
References
1. Bollerslev J, Andersen PE Jr. Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone. 1988; 9(1):7–13.
2. Bollerslev J, Andersen PE Jr. Fracture patterns in two types of autosomal-dominant osteopetrosis. Acta Orthop Scand. 1989; 60(1):110–112.
3. Henriksen K, Gram J, Hoegh-Andersen P, et al. Osteoclasts from patients with autosomal dominant osteopetrosis type I caused by a T253I mutation in low-density lipoprotein receptor-related protein 5 are normal in vitro, but have decreased resorption capacity in vivo. Am J Pathol. 2005; 167(5):1341–1348.
4. Henriksen K, Gram J, Schaller S, et al. Characterization of osteoclasts from patients harboring a G215R mutation in ClC-7 causing autosomal dominant osteopetrosis type II. Am J Pathol. 2004; 164(5):1537–1545.
5. Balemans W, Van Wesenbeeck L, Van Hul W. A clinical and molecular overview of the human osteopetroses. Calcif Tissue Int. 2005; 77(5):263–274.
6. Casden AM, Jaffe FF, Kastenbaum DM, Bonar SF. Osteoarthritis associated with osteopetrosis treated by total knee arthroplasty: report of a case. Clin Orthop Relat Res. 1989; (247):202–207.
7. Strickland JP, Berry DJ. Total joint arthroplasty in patients with osteopetrosis: a report of 5 cases and review of the literature. J Arthroplasty. 2005; 20(6):815–820.
8. de Palma L, Tulli A, Maccauro G, Sabetta SP, del Torto M. Fracture callus in osteopetrosis. Clin Orthop Relat Res. 1994; (308):85–89.
9. Sonohata M, Okubo T, Ono H, Mawatari M, Hotokebuchi T. Bipolar hip arthroplasty for subtrochanteric femoral nonunion in an adult with autosomal dominant osteopetrosis type II. J Orthop Sci. 2011; 16(5):652–655.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download