Abstract
Background
Demineralized bone matrix (DBM) is used for bone healing due to its osteoinductivity, but it requires a carrier for clinical application. Here, we report the effects on the osteoinductivity of DBM by use of a poloxamer 407-based hydrogel as the carrier, compared to sterile water.
Methods
DBM-W and DBM-H represent 27 wt% of DBM with sterile water and DBM with a poloxamer 407-based hydrogel, respectively. Both of the compositions were applied to human mesenchymal stem cell (MSC) cultures, and monitored for alkaline phosphatase (ALP) staining and ALP activity. Six 10-week-old athymic nude rats were used for abdominal muscle grafting with either DBM-W or DBM-H, and were tested by plane radiography, microfocus X-ray computed tomography (CT), and decalcified histology to evaluate ectopic bone formation.
Results
The DBM-W group showed stronger ALP staining at 7, 14, and 21 days of treatment, and significantly higher ALP activity at 7 and 14 days of treatment, compared to the DBM-H group. Plane radiography could not confirm the radio-opaque lesions in the rat ectopic bone formulation model. However, ectopic bone formation was observed in both groups by micro-CT. Compared to the DBM-H group, the DBM-W group showed higher bone volume, percent bone volume and trabecular number, and the difference in percent bone volume was statistically significant. Decalcified histology found bony tissue with lamellation in both groups.
Bone fusion surgery is required for the surgical treatment of degenerative spinal diseases, bone fractures, and tumors. To date, autogenous bone has been considered the most ideal bone graft for bone fusion. However, due to its quantitative limits and donor site morbidity,1) the usage of autogenous bone is gradually decreasing, and various bone graft substitutes have been developed. Bioactive ceramics such as hydroxyapatite, tricalcium phosphate, and calcium pyrophosphate are representative bone graft substitutes which are clinically applied to areas such as spine fusion.2,3,4,5,6) While bioactive ceramics have appealing advantages such as mass production and low risk of inflammation and virus infection, they are osteoconductive materials with a negligible level of osteoinductivity, and thus need to be mixed with autogenous bone for application. To complement this limitation, bone morphogenetic proteins (BMPs) with high osteoinductivity have been developed and applied in various areas, including spinal fusion and tibial non-union.7,8) BMPs are gradually replacing autogenous bone grafts, since they are known to facilitate bone healing and improve fusion rate. However, their many advantages are also accompanied by some disadvantages. They are costly, and are known to cause side effects including ectopic bone formation, formation of seroma,9) neuropathy by neuritis, osteolysis, and soft tissue swelling.10) Moreover, the risk of retrograde ejaculation,11) male infertility and malignancy were also recently reported. Thus, the risk of possible side effects is now advised, rather than their usefulness. Due to its osteoinductivity, demineralized bone matrix (DBM) was frequently applied clinically, before BMP was actively used. However, the usage of DBM is also decreasing, since it has significantly lower osteoinductivity than BMP and mainly functions through osteoconductivity. Thus, it cannot be applied for bone fusion by itself.12) Despite the limitations, use of DBM is still advantageous to reduce autogenous bone graft without using BMP, as DBM rarely develops side effects.
DBM is provided in a powder form, which is hard to pattern and fix to the right position. Thus, it requires various types of carriers such as calcium sulfate, hydroxyapatite, tricalcium phosphate, carboxymethylcellulose, glycerol, hyaluronic acid, and bovine gelatin.
Poloxamer 407 is a tri-block copolymer consisting of a central hydrophobic polypropylene oxide block flanked by two hydrophilic ethylene oxide blocks.13,14) Poloxamer 407 shows thermoreversible properties, forming a gel-like composite at room temperature to facilitate the solubilization of poorly water-soluble drugs, and forming a gel state at body temperature. Poloxamer 407 has been used in the pharmaceutical field because of the properties such as solubilization promotion, stabilization and bio-adhesiveness.14,15)
The putty type of DBM with a carrier improves handling during surgery, but effects on osteoinductivity or osteogenesis have not yet been well examined. This study evaluates the effect on osteogenesis of using poloxamer 407-based hydrogel as a carrier, through various in vitro and in vivo experiments.
DBM-W and DBM-H were synthesized from CG Bio (Seongnam, Korea). DBM-W consists of 27 wt% DBM and 73 wt% sterile water. DBM-H is a putty form mixed with 75 wt% temperature-sensitive poloxamer 407-based hydrogel as a carrier, and 25 wt% DBM.
Alkaline phosphatase (ALP) staining and ALP assay were performed to examine the effects of DBM-W and DBM-H on bone differentiation of messenchymal stem cells (MSCs). 3 × 104 of bone-marrow derived MSCs (BM-MSCs) were seeded on 24-well plates and incubated in a CO2 incubator. After 24 hours of incubation, transwells with either DBM-W or DBM-H were inserted into the plates of BM-MSCs. The treated cells were further incubated with differentiation media including 10 nM dexamethasone, 10 mM beta-glycerophosphate, and 100 µM ascorbic acid for 7, 14, or 21 days. For ALP staining, the differentiation medium was discarded and the cells were washed with 1X Dulbecco's Phosphate-Buffered Saline (DPBS, Life Technology Korea, Seoul, Korea) twice, fixed with 4% paraformaldehyde for 10 minutes, and washed with dH2O. The fixed cells were stained with 0.25% naphthol AS-MX phosphate alkaline solution containing fast blue RR salt (Sigma-Aldrich, Brodby, Denmark) for 10 minutes, washed with dH2O, and subjected to optical microscopy. For ALP assay, the differentiated cells were washed with 1X DPBS (Life Technology Korea) twice and lysed with 500 µL of 0.2% Triton X-100 (Sigma-Aldrich). Equal volumes (20 µL) of p-nitrophenylphosphate and diethanolamine buffer with 0.5 mM MgCl2 (pH 9.8, Sigma-Aldrich) were mixed and reacted for 20 minutes. The reactions were stopped by the addition of 0.2 N NaOH, and monitored by measuring the absorbance at 405 nm with an enzyme-linked immunosorbent assay plate reader.
A total of 6 male athymic nude rats (Hsd:RH-Foxn1, 10 weeks, 280-290 g) were used for this study, with approval from the Standing Ethical Committee at the Laboratory for Animal Research in the Clinical Research Institute of the Seoul National University Hospital (IACUC No. 10-0083). This study followed the 'Guiding Principles for Research Involving Animals and Human Beings' by the American Physiological Society. Rats in this study were given at least a week for adaptation. They were housed in a specific pathogen-free environment with a 12-hour light/12-hour dark cycle at 24℃. Sterile water was available at all times as drinking water, and the animals were fed commercial diets. Three out of 6 rats were randomly selected for the test with DBM-W, and the rest were assigned to the DBM-H group. Zoletil (0.4 mL/kg, Virbac Laboratories, Carros, France) and rompun (10 mg/kg, Bayer Korea Ltd., Seoul, Korea) were used for intraperitoneal anesthesia. After depilation and skin preparation, a longitudinal skin incision was made in the center of the abdominal region. Three pouches were made in the right and left sides of the abdominal muscle. One milliliter of DBM was inserted into the pouches, and the facial layer and skin were sutured (Fig. 1). Immediately after the operation, the rats were intravenously administered with 100 mg of cephazolin, and were then raised without any intervention. Eight weeks later, the animals were sacrificed with an intravenous injection of KCl, after abdominal anesthesia with zoletil and rompun for further tests.
To evaluate the ectopic bone formation, plane radiography was performed under the conditions of a tube to cassette distance of 60 cm, 45 kV, 2.5 mA, and 12 ms for all animal samples. The prepared specimens were subjected to microfocus X-ray computed tomography (Skyscan 1174, Bruker, Kontich, Belgium) with an aluminium filter at 130 kV and 30 µA. Scanned images were reconstructed with sagittal and coronal plane, and the formation of newly calcified tissue was evaluated quantitatively by analyzing the bone surface/volume ratio, percent bone volume, trabecular pattern factor, structure model index, trabecular thickness, trabecular number, trabecular separation, and degree of anisotropy.
The DBM-W group showed stronger ALP staining at 7, 14, and 21 days of treatment, although the staining had faded at 21 days in both of the groups (Fig. 2). ALP activity was also significantly higher in the DBM-W group for the 7- and 14-day treatments (p < 0.001 for DBM-W, p = 0.0003 for DBM-H). The same pattern was observed at 21 days, but no statistical significance was found (p = 0.166).
There were no complications such as infection or death throughout the study with the 6 rats.
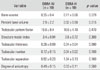
Plane radiography could not detect any clear radio-opaque lesions in either of the groups (Fig. 3). However, micro-CT confirmed distinct calcified tissues in both of the groups by coronal, axial and sagittal imaging (Fig. 4). Quantitative analysis further revealed that the DBM-W group had a higher bone volume and trabecular number, but with no statistical significance. However, the DBM-W group had a significantly higher percentage of bone volume (p = 0.015) than the DBM-H group (Table 1).
Under low magnification, decalcified histology found bony tissue, which was clearly bounded and surrounded by pre-existing abdominal muscle in both of the groups (Fig. 5). Under higher magnification, the lamellate tissue and lacunar space were observed to be arranged concentrically, and a haversian canal was observed in the center. These results indicated that the newly formed calcified tissue was ectopic bone, undergoing remodeling.
DBM requires various types of carrier for handling during clinical application. The typical carriers are ceramics such as glycerol, hyaluronic acid, and calcium phosphate.16) The efficacy of DBM varies based on the level of growth factors such as BMP-2 and BMP-7, thus it is affected by the donor, manufacturing company, vendor, and lot.17,18) One of the reasons for the various efficacies of DBM obtained from different companies could be that each company uses their own carrier.
Both micro-CT and histology confirmed ectopic bone formation in the abdominal muscles of the nude rats from both of the groups. However, the quantitative analysis revealed that the level of newly formed bony tissue was relatively low compared to that obtained with the hydroxyapatite carrier which was used in our previous study.12) Moreover, plane radiography could not confirm the formation of bony tissue. Compared with our previous study using rhBMP-2 in the same ectopic bone formation model, while the rhBMP-2/type I collagen displayed definite ectopic bone formation in 100% of the rats,19) the two kinds of DBM in this study showed only tiny amounts of calcified tissue, not real ectopic bone formation. These data suggest that DBM alone may play a role in extending the bone graft, rather than inducing osteoinductivity.
Given that hydroxyapatite itself is radio-opaque, both DBM-W and DBM-H were considered to induce very weak osteoinductivity based on the ectopic bone formation confirmed through micro-CT and histology, even though the bone formation was not found by plane radiography. DBM facilitates bone healing through osteoconductivity and endochondral osteogenesis, which induces local cell transformation.20) For ectopic bone formation, the quantity and release of growth factors (BMP-2, epidermal growth factor, fibroblast growth factor, platelet-derived growth factor, vascular endothelial growth factor) is more essential than osteoconductivity.
DBM-W showed significantly higher ALP activity, although it contains just 8% more DBM than DBM-H. This implies that the poloxamer 407-based hydrogel itself has no toxicity, but it may inhibit the MSC osteoblastic differentiation by filling up the spaces between the DBM powders, which negatively affects the release of growth factors. Micro-CT of the specimens implanted for 8 weeks showed consistent results. Although there was no statistical significance, the DBM-W group had about 16% higher bone volume, the volumetric parameter of newly formed bony tissue. The DBM-W group also showed 40% higher trabecular number, a parameter describing the quality of the newly formed bony tissue, indicating the effects on the quality of bony tissue as well as quantity. Thus, this study implies that the efficient release of growth factors in DBM is more essential than the quantity of DBM for enhancing osteoblastic differentiation. Collectively, poloxamer 407-based hydrogel induced ectopic bone formation, which represents the efficacy of DBM, but showed lower osteoinductivity compared to sterile water.
The limitation of our study is that the two groups did not contain the same amount of DBM, which introduces the possibility that the results seen could be, at least in part, due to the amount of DBM delivered in the model, regardless of the carrier. However, the osteoinductivity of the DBM-W group was much higher than that of the DBM-H group, the difference of the amount of DBM does not alter the results.
In conclusion, the efficacy of both DBM-W and DBM-H was evaluated by confirming the ectopic bone formation in the two groups. The treatment with DBM-W showed significantly higher MSC osteoblastic differentiation than DBM-H. Both quality and quantity of the ectopic bone were also higher in the DBM-W group, indicating the negative role of the poloxamer 407-based hydrogel on the osteoinductivity of DBM.
Figures and Tables
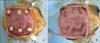 | Fig. 1Operation process. Three incisions and pouches were made per side (right and left) at the abdominal muscle of athymic nude rats. (A) A picture of fixed demineralized bone matrix (DBM). (B) A picture of the sutured pouches after DBM implantation. |
 | Fig. 2Alkaline phosphatase (ALP) staining and ALP activity assay. (A) DBM-W group showed stronger ALP staining at 7, 14, and 21 days of treatment, although the staining faded in both groups at 21 days. (B) ALP activity was significantly higher in the DBM-W group at 7 and 14 days of treatment (p < 0.001 for DBM-W; p = 0.0003 for DBM-H). Although 21 days of treatment showed the same pattern, no statistical significance was found (p = 0.166). DBM: demineralized bone matrix, DBM-W: DBM with sterile water, DBM-H: DBM with poloxamer 407-based hydrogel, D: day. |
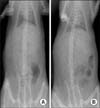 | Fig. 3Anteroposterior abdominal radiographs obtained from DBM-W group (A) and DBM-H group (B) after 8 weeks of implantation. Easily identifiable radio-opaque lesions were not observed in either group. DBM: demineralized bone matrix, DBM-W: DBM with sterile water, DBM-H: DBM with poloxamer 407-based hydrogel. |
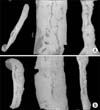 | Fig. 4Microfocus X-ray computed tomography of a specimen from the DBM-W group (A) and DBM-H group (B) after 8 weeks of implantation. For both A and B, each panel represents a transaxial image, sagittal image, and coronal image (from left to right). DBM: demineralized bone matrix, DBM-W: DBM with sterile water, DBM-H: DBM with poloxamer 407-based hydrogel. |
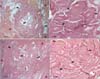 | Fig. 5Decalcified histology of the rats in the DBM-W group (A) and DBM-H group (B) after 8 weeks of implantation (H&E, × 12.5 and × 100). Under low magnification, both of the groups showed newly formed bone surrounded by abdominal muscle. Under higher magnification, the lamellate tissue and lacunar space were arranged concentrically, and a haversian canal was observed in the center, confirming bone remodeling. DBM: demineralized bone matrix, DBM-W: DBM with sterile water, DBM-H: DBM with poloxamer 407-based hydrogel, arrow heads: pre-existing abdominal muscle, arrows: newly formed ectopic calcified tissue. |
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid (No. 03-2012-150) from the Seoul National University Hospital Research Fund.
References
1. Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989; 71(4):677–680.
2. Lee JH, Hwang CJ, Song BW, Koo KH, Chang BS, Lee CK. prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009; 90(3):804–810.
3. Gunzburg R, Szpalski M. Use of a novel beta-tricalcium phosphate-based bone void filler as a graft extender in spinal fusion surgeries. Orthopedics. 2002; 25:5 Suppl. s591–s595.
4. Boden SD, Martin GJ Jr, Morone M, Ugbo JL, Titus L, Hutton WC. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine (Phila Pa 1976). 1999; 24(4):320–327.
5. Lee JH, Chang BS, Jeung UO, Park KW, Kim MS, Lee CK. The first clinical trial of beta-calcium pyrophosphate as a novel bone graft extender in instrumented posterolateral lumbar fusion. Clin Orthop Surg. 2011; 3(3):238–244.
6. Lee JH, Chang BS, Ryu HS, Lee CK. A 90-day subchronic toxicity study of beta-calcium pyrophosphate in rat. Drug Chem Toxicol. 2009; 32(3):277–282.
7. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004; 4(5):527–538.
8. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976). 2002; 27(23):2662–2673.
9. Garrett MP, Kakarla UK, Porter RW, Sonntag VK. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery. 2010; 66(6):1044–1049.
10. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011; 11(6):471–491.
11. Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011; 11(6):511–516.
12. Lee JH, Lee KM, Baek HR, Jang SJ, Lee JH, Ryu HS. Combined effects of porous hydroxyapatite and demineralized bone matrix on bone induction: in vitro and in vivo study using a nude rat model. Biomed Mater. 2011; 6(1):015008.
13. Ha NS, Tran TT, Tran PH, Park JB, Lee BJ. Dissolution-enhancing mechanism of alkalizers in poloxamer-based solid dispersions and physical mixtures containing poorly water-soluble valsartan. Chem Pharm Bull (Tokyo). 2011; 59(7):844–850.
14. Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006; 23(12):2709–2728.
15. Yu CH, Lee JH, Baek HR, Nam H. The effectiveness of poloxamer 407-based new anti-adhesive material in a laminectomy model in rats. Eur Spine J. 2012; 21(5):971–979.
16. Qiu QQ, Shih MS, Stock K, et al. Evaluation of DBM/AM composite as a graft substitute for posterolateral lumbar fusion. J Biomed Mater Res B Appl Biomater. 2007; 82(1):239–245.
17. Bae H, Zhao L, Zhu D, Kanim LE, Wang JC, Delamarter RB. Variability across ten production lots of a single demineralized bone matrix product. J Bone Joint Surg Am. 2010; 92(2):427–435.
18. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006; 31(12):1299–1306.
19. Lee JH, Jang SJ, Koo TY, et al. Expression, purification and osteogenic bioactivity of recombinant human BMP-2 derived by Escherichia coli. Tissue Eng Regen Med. 2011; 8(1):8–15.
20. Lin X, Pena LA, Zamora PO, Campion SL, Takahashi K. Augmentation of demineralized bone matrix (DBM) mineralization by a synthetic growth factor mimetic. J Orthop Res. 2006; 24(11):2051–2058.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download