Abstract
Background
We investigated the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in malignant fibrous histiocytoma (MFH), and determined whether these could be useful as prognostic factors.
Methods
Among patients treated from 1993 to 2007, 30 cases of MFH were evaluated. Immunohistochemical staining was performed for MMP-2, MMP-9, TIMP-1, and TIMP-2 using paraffin wax-embedded blocks of MFH tissues. Reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot and zymography were performed using fresh tissues obtained from 17 of the 30 cases. The levels of MMP and TIMP expression were compared between the MFH and normal control groups, and between non-metastatic and metastatic MFH groups.
Results
Expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were higher in the MFH group than the control group by RT-PCR, Western blotting, and zymography. Immunohistochemical staining revealed that MMP-2 and MMP-9 protein expression was higher in the metastatic than in the non-metastatic group. The expression levels of MMP-2 and TIMP-1 were significantly higher in the metastatic than in the non-metastatic group (p < 0.05) by RT-PCR. By Western blot analysis, the expression levels of MMP-2, TIMP-1, and TIMP-2 were higher in the metastatic group (p < 0.05), but MMP-9 showed only a slight increase in the metastatic group compared with the non-metastatic group (p > 0.05). Finally, gelatin zymography analysis showed that the expression levels of the pro- and active forms of MMP-2 were significantly higher in the metastatic group (p < 0.05), but the expression of the pro- and active forms of MMP-9 showed a slight decrease in the metastatic group (p > 0.05).
Conclusions
These results suggest that MMP-2, MMP-9, TIMP-1, and TIMP-2 may have important roles in the development and progression of MFH, and that the degree of expression of these metalloproteinases and their inhibitors, especially MMP-2, could be useful as prognostic factors related to metastasis in MFH.
Malignant fibrous histiocytoma (MFH) is the most common soft tissue sarcoma of late adult life,1,2,3,4) and most cases occur in persons aged between 50 and 70 years.4) MFH occurs most frequently on the lower extremity, especially the thigh, followed by the trunk, upper extremities, and retroperitoneum.5,6,7) MFH is a fully malignant sarcoma and has a broad range of histological appearances, which may be divided into the following subtypes: storiform-pleomorphic, myxoid, giant-cell, and inflammatory. However, these histological variants do not correlate with prognosis.5) Recent large-scale studies have shown local recurrence rates from 19% to 44%, distant metastatic rates from 23% to 36%, and 5-year survival rates from 39% to 74%.5,6,7,8,9) Tumor size, depth, and histological grade are the most important prognostic factors.5,6,7,8,9) However, despite intensive investigation, progress in the management of MFH has been minimal and its biological features remain unknown.
Malignant tumor cell invasion is believed to involve a complex series of events, consisting of tumor cell adhesion, extracellular matrix (ECM) proteolysis, and cell migration within the microenvironment. In particular, the matrix metalloproteinases (MMPs) are known to participate in the degradation of the ECM and basement membrane.10,11,12) To date, more than 20 MMP genes have been identified, and these have been classified as collagenases, gelatinases, stromelysins, membrane-type MMPs, and others, according to their structures and substrate specificities.13) Of these MMPs, the gelatinases, MMP-2 and MMP-9, degrade type IV collagen, the major components of the basement membrane. Furthermore, these two MMPs are most consistently overexpressed in cancer tissue, and their upregulation has been associated with tumor aggressiveness, metastatic potential, and poor prognosis.11,14,15,16,17) The activation of the latent pro-enzymes, pro-MMP-2 and pro-MMP-9, is regulated by tissue inhibitor of metalloproteinase (TIMP) 2 and TIMP-1, respectively, which form noncovalent complexes with MMPs.1,2) Also, these pro-MMPs are converted to their active forms proteolytically by the cleavage of a propeptide domain.
There have been many reports about the expression of MMPs in cancer tissues, but few in sarcomas. In this study, we evaluated the expression of MMP-2, MMP-9, TIMP-2, and TIMP-1 in MFH tissues, and assessed whether their expression levels correlated with metastasis potential.
From 1993 to 2007, 101 patients (58 males and 43 females) were diagnosed with MFH at our hospital. Among them, 30 patients were included in this study. Inclusion in this study was based upon the availability of a fresh MFH tissue sample. Additional criteria included availability of full clinical data and duration of follow-up over 2 years or until death. There were 14 men and 16 women, and their demographic data are shown in Table 1. There were 21 disease-free patients, no case of metastasis in non-metastatic MFH patients, and nine disease-related deaths by the time we finished this article. Thirty cases were evaluated in an immunohistochemical study, and 17 of the 30 were also evaluated by molecular genetic studies: reverse transcriptase polymerase chain reaction (RT-PCR), Western blotting, and zymography. Four tissue samples of normal muscle and fascia were used as controls.
The all cases that underwent the immunohistochemical study were divided into two groups. The first was 20 cases of MFH with no metastasis (non-metastatic group), and the second was 10 cases of MFH with metastasis (metastatic group). The twenty-one cases that underwent molecular genetic studies were also divided into three groups: 12 cases in the non-metastatic group, five in the metastatic group, and four in the control group.
All procedures for the immunohistochemical staining were performed with the MicroProbe staining system based on capillary action.18) Immunohistochemical staining was performed with the Cap-Plus Detection Kit (Zymed Laboratories, South San Francisco, CA, USA). Paraffin wax sections, 4-µm-thick, mounted on probe slides, were immunostained with anti-mouse monoclonal antibodies for MMP-2, MMP-9, TIMP-1, and TIMP-2 antigens (Oncogene, La Jolla, CA, USA) by the avidin-biotin peroxidase complex method. Sections were deparaffinized and immersed in 60% methanol with 0.3% hydrogen peroxide for 7 minutes to block endogenous peroxidase activity, then incubated with blocking solution (Zymed Laboratories) for 3 minutes at 45℃. The sections were incubated with the anti-mouse monoclonal antibodies to MMP-2, TIMP-1, and TIMP-2 (1:50) for 2 hours at room temperature in a humidified chamber. For the primary mouse monoclonal antibodies to MMP-9, sections were treated with citrate buffer, pH 6.0, at 85℃ for 15 minutes and incubated with MMP-9 antibodies (1:25) overnight at 4℃. Biotin-labeled secondary antibody (Zymed Laboratories) was added for 7 minutes at 45℃. The streptavidin-horseradish peroxidase (Zymed Laboratories) was then applied to the capillary channels, and incubated for 7 minutes at 45℃. After drainage, the tissue sections were ready for chromogen reaction with 3-amino-9-ethylcarbazole (AEC, Zymed Laboratories). The sections were counterstained with hematoxylin and mounted in Universal Mount (Research Genetics, Huntsville, AL, USA).
Immunohistochemical staining was evaluated by scoring the pattern and staining intensity of the cells under a ×100 light microscope. The staining level was classified into four classes according to the distribution of stained cells under the light microscope as 0%, < 25%, 25%-50%, and > 50% of total tumor cells, classified as none, mild, moderate, and diffuse, respectively. Two specialists assessed the staining level of each case twice to determine interobserver and intraobserver reliability. Observer agreement was then assessed with the unweighted kappa coefficient (κ).
Malignant fibrous histiocytoma tissue fragments were acquired at surgery. Tissue was flash-frozen in liquid nitrogen. Total RNA from the tissue was extracted using the methodology for TRIzol reagent (Invitrogen, San Diego, CA, USA). RNA (1 µg) was reverse-transcribed using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen).
Primers for MMP-2, MMP-9, TIMP-1, TIMP-2, and actin, a housekeeping gene that is expressed constitutively, were designed. All primers were checked against the GenBank database to ensure no similarities with other known human DNA sequences. The primer sequences are shown in Table 2. The polymerase chain reaction (PCR) reaction mixture contained 2 µL of each cDNA sample, 10 pM each of sense and antisense primers, and other PCR reagents in a final volume of 20 µL. PCR reagents, dNTP, Taq DNA polymerase, and 10× reaction buffer (40 mM KCl, 10 mM Tris-HCl, pH 9.0, 1.5 mM MgCl2, stabilizer, and tracking dye) obtained from Accupower PCR PreMix (Bioneer, Daejeon, Korea). PCR reactions were conducted using a Perkin-Elmer GeneAmp PCR system 2700 (Perkin-Elmer, Norwalk, CT, USA) as follows. PCR cycles were 94℃ for 5 minutes, then 35 cycles of denaturation at 94℃ for 1 minute, annealing at 60℃ (65℃ for MMP-9) for 1 minute, and polymerization at 72℃ for 2 minutes, followed by 72℃ for 7 minutes.
The RT-PCR products were visualized on 1% agarose gels electrophoresed in 0.5× TBE buffer containing 0.5 µg/mL ethidium bromide. The fidelity of the RT-PCR product was verified by comparing the size of the amplified products to the expected cDNA bands and by sequencing the PCR products. With a housekeeping gene, β-actin, as an internal control, expression levels of MMP-2 and MMP-9 genes were analyzed by semi-quantitative RT-PCR using Image-Pro Plus (Media Cybernetics, Bethesda, MD, USA).
To investigate whether TIMP-1 and TIMP-2 could inhibit the expression of MMP-2 and MMP-9 in human MFH cells, protein levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were determined by Western blotting. Samples were homogenized in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% NaDOC, 50 mM Tris, pH 8.0, 1 mM PMSF, and 1 µg/mL aprotinin and leupeptin) and the supernatant was collected following centrifugation. Protein concentrations were determined by the bicinchoninic acid method (BCA kit, Pierce, Rockford, IL, USA). Equal amounts of proteins of MFH cell were electrophoresed in 10% sodium dodecyl sulfate (SDS) polyacrylamide gel using a MiniProtean (Bio-Rad, Hercules, CA, USA) gel apparatus, and the electrophoresed gel slabs were electrotransferred to nitrocellulose membranes (Amersham Life Science, Buckinghamshire, UK) overnight at 4℃ in transfer buffer (30 mM Tris, 240 mM glycine, 25% methanol) at a constant voltage of 20 V. The membrane was blocked in 5% defatted milk powder in TBS-T, pH 7.6 (100 mM Tris buffer with 150 mM NaCl and 0.1% Tween 20), and the blot was incubated with specific primary antibodies. To detect MMP-2, MMP-9, TIMP-1, TIMP-2, and β-actin, we used monoclonal antibodies (Oncogene) at a final concentration in blocking buffer of 1 µg/mL (1:1,000 dilution). These antibodies recognize both the latent (92 kDa) and active forms (82 kDa) of MMP-9, and the latent (72 kDa) and active forms (62 kDa) of MMP-2. Horseradish peroxidase goat anti-mouse immunoglobulin (BD Bioscience, San Jose, CA, USA) was used as the secondary antibody (1:2,000) for 1 hour at room temperature, and then the membrane was washed four times for 15 minutes each in TBS-T. Bands were developed using the Enhanced Chemiluminescence (ECL Plus) system (Amersham Bioscience, Buckinghamshire, UK). The MMP-2 and MMP-9 were semi-quantified using an Image-Pro Plus (Media Cybernetics).
This is an in vitro assay using gelatin-substrate gel electrophoresis to measure the level of MMP activity in MFH samples. Frozen MFH tissues were pulverized in liquid nitrogen and homogenized in buffer (50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 200 mM NaCl) and a homogenizer. Protein concentrations were determined by the BCA method (BCA kit, Pierce). Samples were mixed with an equal volume of 4× sample buffer (200 mM Tris-HCl, 8% SDS, 0.4% bromophenol blue, 40% glycerol). Samples were electrophoresed on 8% SDS polyacrylamide gels containing 2 mg/mL gelatin (type A, Sigma, St. Louis, MO, USA). Following electrophoresis, the gel was washed three times for 30 minutes in 2.5% Triton X-100 at room temperature, and incubated for 18 hours at 37℃ in incubation buffer (50 mM Tris-HCl, pH 7.5, 5 mM CaCl2, 200 mM NaCl). The gel was stained for 1 hour with Coomassie Brilliant Blue R-250 (0.2% Coomassie Brilliant Blue R-250, 20% methanol, 10% acetic acid in H2O) and destained in washing solution (30% methanol, 10% acetic acid). White bands on the blue background indicated zones of digestion corresponding to the presence of different pro-MMPs and activated MMPs on the basis of their molecular weight. The MMP-2 and MMP-9 were semi-quantified using Image-Pro Plus (Media Cybernetics).
Intensities of bands on images were quantitated with the Multi Gauge ver. 3.0 (Fuji Film, Tokyo, Japan) and Scion Image. The relationship between the expression of MMP/TIMP and distant metastasis was examined. Statistical significance was determined at p < 0.05 (Fisher exact test). To analyze the association and correlation between metastasis and the expression level of MMP and TIMP; it was analyzed statistically by multiple regression analysis. We used the SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
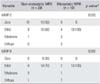
Immunohistochemical staining was done for MMP-2, MMP-9, TIMP-1, and TIMP-2 (Fig. 1). For MMP-2 in the non-metastatic group, 10 cases showed no expression, nine mild expression, and one moderate expression. The expression rate of MMP-2 in the non-metastatic MFH group was 50% (10 cases). The metastatic group showed four with mild expression, three with moderate expression, and three with diffuse expression. The expression rate of MMP-2 in the metastatic group was 100% (10 cases; p < 0.05). For MMP-9 in the non-metastatic group, six showed no expression, eight mild expression, five moderate expression, and one diffuse expression. The expression rate of MMP-9 in the non-metastatic group was 70% (14 cases). The metastatic group showed two cases of mild expression, one moderate expression, and seven diffuse expression (p < 0.05) (Table 3). The expression rate of MMP-9 in the metastatic group was 100% (10 cases; p < 0.05). The expression rates of TIMP-1 and TIMP-2 are shown in Table 4.
Multiple regression analysis showed that MMP-2 and MMP-9 were associated with metastasis significantly (p < 0.05). However, there was no statistical significance for TIMP-1 or TIMP-2 (Table 5).
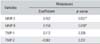
RT-PCR results showed that expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in both the non-metastatic and metastatic groups were significantly higher than in the control group (p < 0.05). The expression levels of MMP-2 and TIMP-1 were significantly higher in the metastatic group than in the non-metastatic group (p < 0.05). However, the expression levels of MMP-9 and TIMP-2 were lower in the metastatic group than in the non-metastatic group (Fig. 2).
The Western blotting results also showed that the expressions of MMP-2, MMP-9, TIMP-1, and TIMP-2 were higher in the non-metastatic and metastatic groups than in the control group, and these results were significant for MMP-9 and TIMP-2 (p < 0.05). The expression levels of MMP-2, TIMP-1, and TIMP-2 were significantly higher in the metastatic group than the non-metastatic group (p < 0.05), but there was no statistically significant difference in the expression of MMP-9 between two groups (p > 0.05) (Fig. 3).
Gelatin zymography revealed that the activities of the pro- and active forms of MMP-2 and MMP-9 in the non-metastatic and metastatic groups were higher than in the control group (p < 0.05). The expression levels of the pro- and active forms of MMP-2 were significantly higher in the metastatic group than in the non-metastatic group (p < 0.05), but the expression levels of pro- and active forms of MMP-9 decreased slightly in the metastatic group (p > 0.05) (Fig. 4).
The MMPs are generally present in greater amounts and are more often activated in and around malignant cancers than in normal, benign, or premalignant tissues, and are most highly expressed in areas of active invasion at the tumor-stroma interface.11,18,19,20,21,22,23,24) Furthermore, significant positive correlations have been found between MMP expression and various indicators of poor prognosis in virtually all types of cancer, and in some cases, MMP upregulation has been identified as an independent predictor of shortened disease-free and overall survival.11,14,15,16,17)
The TIMPs have seemingly paradoxical effects. They are known to suppress metastasis and preserve ECM integrity in vitro25) and in experimental animals.26) However, recent studies have shown that increased levels of TIMP-1 in human cancer tissues and in plasma are associated with a poor prognosis.18,19,27) Furthermore, plasma TIMP-1 levels have been reported to be higher in patients with osteosarcoma than in normal controls, particularly in patients who later developed metastasis and/or local recurrence and in those with metastasis at diagnosis.28)
These findings may reflect an attempt to control the increased proteolytic activity induced by MMPs, but there is increasing evidence that the TIMPs are multifunctional proteins. For example, TIMP-2 is required for the activation of MMP-2, and TIMP-1 has been reported to act as a growth factor.
TIMP-1 may confer a growth advantage by upregulating vascular endothelial growth factor (VEGF) expression in vivo. Indeed, TIMP-1 transfected human and rat mammary cancer cells show a direct relationship between TIMP-1 levels and VEGF production, tumor growth, and tumor vascularization and proliferation in vivo.29)
TIMP-2 participates in both the activation and inhibition of MMP-2 in a dose-dependent manner. Thus, increasing levels of TIMP-2 should increase MMP-2 activation, but at some stage the activation and proteolytic activity of MMP-2 are blocked by the inhibitory activity of TIMP-2.20)
The C-terminal domain of TIMP-1 binds the hemopexin domain of MMP-9 more readily than other MMPs, whereas the C-terminal domain of TIMP-2 preferentially binds the hemopexin domain of MMP-2.21) Although TIMPs have dual functions, that is, MMP inhibition and growth factor-like activity, during the regulation of tumor invasion, their tumor invasion-inhibitory activities have been identified more frequently.
Himelstein et al.24) found intense MMP-9 expression in most tumor cells in all samples of pretreatment childhood osteosarcoma, and that the number of positively stained cells decreased after treatment. Additionally, they found intense MMP-9 expression in most metastatic lesions. Ferrari et al.28) performed an immunohistochemical study on high-grade osteosarcomas, and detected a high percentage of tumors with moderate MMP-9 expression, especially in the patients who developed metastasis during follow-up. Based on these results, they proposed that MMP-9 had potential prognostic value during the early phase of osteosarcoma progression.
However, the associations between MMP/TIMP ratios and prognosis are controversial. Maguire et al.30) reported that neither ratios of MMP-2 and MMP-9 mRNAs versus their inhibitors nor active MMP-2 protein levels in soft tissue sarcoma specimens correlated with distant metastasis. They concluded that gelatinases and their inhibitors had limited value as predictors of distant metastasis in soft tissue sarcoma.
A small number of studies have reported the expression of MMPs and TIMPs in MFH. In 1993, Soini et al.22) reported that the synthesis of MMP-2 and MMP-9 mRNAs was quantitatively similar for MFHs and their benign counterparts (dermatofibromas), suggesting that there is no correlation between the biological behavior of tumors and the syntheses of MMP-2 or MMP-9 mRNA. They proposed one mechanism that could account for this result, namely, that the balance between metalloproteinases and inhibitors, and not absolute levels of proteinases, were importantly related to tumor invasive properties. However, in another study, they reported that the neoplastic cells of MFHs showed markedly higher levels of TIMP-1 and TIMP-2 mRNA expression than dermatofibromas.23)
MMP-2 is secreted from cells as a zymogen, pro-MMP-2. Its activation is regulated by MMP-14, depending on the presence of low concentrations of the inhibitor TIMP-2. Thus, we evaluated immunohistochemical staining for MMP-14 to assess the correlation between MMP-2, TIMP-2, and MMP-14 expression in MFH. However, the results showed no statistically significant difference for MMP-14 (Table 6, Fig. 5). This suggests that MMP-14 may act in the first steps of tumor cell invasion and metastasis. In the present study, the expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were higher in MFH group than the control group by RT-PCR, Western blotting, and zymography. Furthermore, the expression levels of MMP-2 and MMP-9 in the metastatic group were significantly higher than in the non-metastatic group by immunohistochemistry. Especially, MMP-2 expression and MMP-2 activity were higher in the metastatic group than the non-metastatic group consistently in all studies, with statistical significance.
These results suggest that MMP-2, MMP-9, TIMP-1, and TIMP-2 may have important roles in the development and progression of MFH, and that the degree of expression of these metalloproteinases and their inhibitors, especially MMP-2, could be useful as a prognostic factor related to metastasis in MFH. In conclusion, we consider that MMP-2, MMP-9, TIMP-1, and TIMP-2 may be associated with the development and progression of MFH, although the exact roles of these proteins remain unclear. Also, we propose a possible role for MMP-2 expression in predicting metastasis and poor prognosis in MFH.
Figures and Tables
Fig. 1
Immunohistochemical staining findings for matrix metalloproteinase (MMP) 2, MMP-9, tissue inhibitors of metalloproteinase (TIMP) 1, and TIMP-2 in the non-metastatic malignant fibrous histiocytoma (MFH) and metastatic MFH cells. MMP-2 and MMP-9 were weakly expressed in non-metastatic MFH cells (A, C), but predominantly expressed in metastatic MFH cells with diffuse, strong intensities (B, D). TIMP-1 and TIMP-2 showed negative expressions in non-metastatic MFH cells (E, G) and focal strong expression in metastatic MFH cells (F, H) (A-H, ×200). (A) MMP-2 in non-metastatic MFH. (B) MMP-2 in metastatic MFH. (C) MMP-9 in non-metastatic MFH. (D) MMP-9 in metastatic MFH. (E) TIMP-1 in non-metastatic MFH. (F) TIMP-1 in metastatic MFH. (G) TIMP-2 in non-metastatic MFH. (H) TIMP-2 in metastatic MFH.

Fig. 2
The results of reverse transcriptase polymerase chain reaction for matrix metalloproteinase (MMP) 2, MMP-9, tissue inhibitors of metalloproteinase (TIMP) 1, and TIMP-2. The expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the non-metastatic malignant fibrous histiocytoma (MFH) and metastatic MFH groups were significantly higher than in the control group. The expression levels of MMP-2 and TIMP-1 were significantly higher and the expression levels of MMP-9 and TIMP-2 were lower in the metastatic MFH group than in the non-metastatic MFH group (p < 0.05). Meta: metastatic. *Significant, Mann-Whitney U-test, p < 0.05.
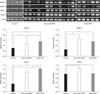
Fig. 3
Western blotting results for matrix metalloproteinase (MMP) 2, MMP-9, tissue inhibitors of metalloproteinase (TIMP) 1, and TIMP-2. The expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the non-metastatic malignant fibrous histiocytoma (MFH) and metastatic MFH groups were higher than those in the control group, and these results were significant for MMP-9 and TIMP-2 (p < 0.05). The expression levels of MMP-2, TIMP-1, and TIMP-2 were higher in the metastatic MFH group than in the non-metastatic MFH group (p < 0.05). Meta: metastatic. *Significant, Mann-Whitney U-test, p < 0.05.
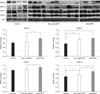
Fig. 4
Enzyme activity through gelatin zymography. Activities of the active forms of matrix metalloproteinase (MMP) 2 and MMP-9 were greater in the non-metastatic malignant fibrous histiocytoma (MFH) and metastatic MFH groups than in the control group. The expression levels of the pro- and active forms of MMP-2 were significantly higher and the expression levels of the pro- and active forms of MMP-9 decreased slightly in the metastatic MFH group than in the non-metastatic MFH group. Meta: metastatic. *Significant, Mann-Whitney U-test, p < 0.05.
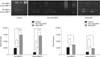
Fig. 5
Results of reverse transcriptase polymerase chain reaction (A) and Western blotting (B) for matrix metalloproteinase (MMP) 14. Expression levels of MMP-14 in the control, non-metastatic malignant fibrous histiocytoma (MFH), and metastatic MFH groups were not statistically significantly different. Meta: metastatic. *Significant, Mann-Whitney U-test, p < 0.05.

ACKNOWLEDGEMENTS
This study was supported by a grant (CRI09053-1) from Chonnam National University Hospital Biomedical Research Institute.
References
1. Bertoni F, Capanna R, Biagini R, et al. Malignant fibrous histiocytoma of soft tissue: an analysis of 78 cases located and deeply seated in the extremities. Cancer. 1985; 56(2):356–367.
2. Enzinger FM. Malignant fibrous histiocytoma 20 years after Stout. Am J Surg Pathol. 1986; 10:Suppl 1. 43–53.
3. Le Doussal V, Coindre JM, Leroux A, et al. Prognostic factors for patients with localized primary malignant fibrous histiocytoma: a multicenter study of 216 patients with multivariate analysis. Cancer. 1996; 77(9):1823–1830.
4. Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978; 41(6):2250–2266.
5. Belal A, Kandil A, Allam A, et al. Malignant fibrous histiocytoma: a retrospective study of 109 cases. Am J Clin Oncol. 2002; 25(1):16–22.
6. Gibbs JF, Huang PP, Lee RJ, et al. Malignant fibrous histiocytoma: an institutional review. Cancer Invest. 2001; 19(1):23–27.
7. Peiper M, Zurakowski D, Knoefel WT, Izbicki JR. Malignant fibrous histiocytoma of the extremities and trunk: an institutional review. Surgery. 2004; 135(1):59–66.
8. Hsu HC, Huang EY, Wang CJ. Treatment results and prognostic factors in patients with malignant fibrous histiocytoma. Acta Oncol. 2004; 43(6):530–535.
9. Salo JC, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Malignant fibrous histiocytoma of the extremity. Cancer. 1999; 85(8):1765–1772.
10. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993; 9:541–573.
11. Sato H, Takino T, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci. 2005; 96(4):212–217.
12. Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006; 25(1):35–43.
13. Johansson N, Ahonen M, Kahari VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000; 57(1):5–15.
14. Koga K, Nabeshima K, Aoki M, et al. Emmprin in epithelioid sarcoma: expression in tumor cell membrane and stimulation of MMP-2 production in tumor-associated fibroblasts. Int J Cancer. 2007; 120(4):761–768.
15. Liu J, Zhan M, Hannay JA, et al. Wild-type p53 inhibits nuclear factor-kappaB-induced matrix metalloproteinase-9 promoter activation: implications for soft tissue sarcoma growth and metastasis. Mol Cancer Res. 2006; 4(11):803–810.
16. Scapolan M, Perin T, Wassermann B, et al. Expression profiles in malignant fibrous histiocytomas: clues for differentiating 'spindle cell' and 'pleomorphic' subtypes. Eur J Cancer. 2008; 44(2):298–309.
17. Roebuck MM, Helliwell TR, Chaudhry IH, et al. Matrix metalloproteinase expression is related to angiogenesis and histologic grade in spindle cell soft tissue neoplasms of the extremities. Am J Clin Pathol. 2005; 123(3):405–414.
18. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000; 18(5):1135–1149.
19. Schrohl AS, Holten-Andersen MN, Peters HA, et al. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res. 2004; 10(7):2289–2298.
20. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001; 17:463–516.
21. Murphy G, Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 1995; 248:496–510.
22. Soini Y, Salo T, Oikarinen A, Autio-Harmainen H. Expression of 72 kilodalton and 92 kilodalton type IV collagenase in malignant fibrous histiocytomas and dermatofibromas. Lab Invest. 1993; 69(3):305–311.
23. Hurskainen T, Soini Y, Tuuttila A, Hoyhtya M, Oikarinen A, Autio-Harmainen H. Expression of the tissue metal-loproteinase inhibitors TIMP-1 and TIMP-2 in malignant fibrous histiocytomas and dermatofibromas as studied by in situ hybridization and immunohistochemistry. Hum Pathol. 1996; 27(1):42–49.
24. Himelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol. 1998; 31(6):471–474.
25. Albini A, Melchiori A, Santi L, Liotta LA, Brown PD, Stetler-Stevenson WG. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991; 83(11):775–779.
26. Reich R, Thompson EW, Iwamoto Y, et al. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988; 48(12):3307–3312.
27. Holten-Andersen M, Christensen IJ, Nilbert M, et al. Association between preoperative plasma levels of tissue inhibitor of metalloproteinases 1 and rectal cancer patient survival. a validation study. Eur J Cancer. 2004; 40(1):64–72.
28. Ferrari C, Benassi S, Ponticelli F, et al. Role of MMP-9 and its tissue inhibitor TIMP-1 in human osteosarcoma: findings in 42 patients followed for 1-16 years. Acta Orthop Scand. 2004; 75(4):487–491.
29. Yoshiji H, Harris SR, Raso E, et al. Mammary carcinoma cells over-expressing tissue inhibitor of metalloproteinases-1 show enhanced vascular endothelial growth factor expression. Int J Cancer. 1998; 75(1):81–87.
30. Maguire PD, Qi W, Lallemand R, Scully SP. Gelatinase and inhibitor expression in soft tissue sarcomas: lack of correlation with distant metastasis. Oncology. 2000; 59(2):139–144.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download