Abstract
Background
We intended to clarify the hypothesis that minimally invasive total hip arthroplasty (MI-THA) leads to less tissue damage and inflammatory response than does conventional total hip arthroplasty (C-THA).
Methods
We performed 30 cases of THA between September 2005 and May 2006 and evaluated these cases prospectively. We chose 15 MI-THA cases for the study group and another 15 C-THA cases for the control group. We checked skeletal muscle marker enzymes, such as serum creatinine kinase and aldolase, the pro-inflammatory cytokines, interleukin (IL)-6 and 8, and the anti-inflammatory cytokines, IL-10 and IL-1 receptor antagonist (ra) the day before surgery and at postoperative days 1, 7, and 14.
Minimally invasive total hip arthroplasty (MI-THA) is the concept that a small skin incision can minimize the tissue damage during THA and that the minimized damage allows the patient to recover easier. The indications for MI-THA have become wider in recent years with advances in surgical equipment, imaging modalities, and surgical techniques. Compared with the conventional surgery, MI-THA shows clinically effective results for reducing pain, lowering the volume of blood lost and blood transfusions, achieving prompt rehabilitation, and obtaining a satisfactory cosmetic outcome after surgery.1,2,3,4,5,6) However, there is concern about possible tissue damage or larger soft tissue injury following MI-THA than that in conventional THA (C-THA) because of excessive traction and compression of subcutaneous tissue and muscle. However, the number of MI-THA cases continually increases, and the number of reports with good clinical results following MI-THA rather that C-THA has increased as well. Few quantitative studies have identified an effect of MI-THA on soft tissue damage in human subjects. Thus, we designed a comparative study to examine the differences in the inflammatory reaction between patients who underwent MI-THA and those who received C-THA to test the hypothesis that MI-THA is less damaging than C-THA.
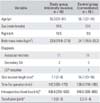
Fifteen of 30 candidates were selected as the MI-THA study group to compare with another 15 as the control C-THA group. No differences in age, sex, body mass index, surgical site, diagnosis, or other demographics were observed between the two groups (Table 1). Patients with a medical history of endocrine, cardiopulmonary, hepatobiliary, renal or autoimmune diseases were excluded. Patients stopped non-steroidal anti-inflammatory drugs at least 6 weeks before the study to minimize the effect on inflammatory cytokines. We replaced the drugs with non-opioid analgesic drugs such as acetaminophen or tramadol. After surgery, we controlled pain with a Patient-controlled analgesia system. All surgical procedures were performed by a single operator (YJC) under general anesthesia using the posterior modified Gibson approach with the pateint in the lateral decubitus position. The operator used a 15-cm skin incision through the surface of the greater trochanter in the control group. Subcutaneous tissue, the iliotibial band, and the gluteus maximus were dissected and retracted. The piriformis and short external rotators were completely detached from the greater trochanter. An approach was made to the hip joint. Finally, the femoral head was exposed at the hip.7) The operator made a skin incision along the long axis of the femur at a ratio of 1/3 the length of the skin incision superiorly and 2/3 inferiorly from the tip of the greater trochanter in MI-THA study group, after considering body weight and the degree of obesity. The length of the skin incision was 7-8 cm. The piriformis were completely detached, the short external rotators were partially dissected in the vicinity of the greater trochanter, and the femoral head was posteriorly dislocated. In both groups, the posterior joint capsule, piriformis muscle, and short external rotators were sutured with non-absorbable thread by making a small hole in the greater trochanter after implanting the prosthesis.
The length of the skin incision, operation time, intraoperative blood loss, and postoperative blood transfusion volume were evaluated between the groups. A visual analog pain scale was recorded. Serological markers indicating muscle injury, including creatinine kinase (CK) and aldolase, the pro-inflammatory cytokines interleukin (IL)-6 and IL-8, and the anti-inflammatory cytokines IL-10 and IL-1 receptor antagonist (ra) were measured to assess the degree of tissue injury and the systemic inflammatory reaction. All evaluations were performed at 7:00 AM prior to the operation and on postoperative days (POD) 1, 3, 7, and 14. Serum CK was analyzed by the ultraviolet kinetic method with an automatic chemical analyzer (Hitachi Inc., Tokyo, Japan), and serum aldolase was analyzed by the ultraviolet kinetic method with a spectrophotometer (Sentinel, Rome, Italy). The cytokines were analyzed with a commercially available enzyme linked immunosorbent assay test kit (Quantikine, R&D Systems, Minneapolis, MN, USA). Results were analyzed with the Mann-Whitney U-test and SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Statistical significance was tested with a 95% confidence interval. A p < 0.05 was considered significant.
Mean length of the skin incision was 7.7 ± 0.5 cm in the study group and 14.7 ± 1.3 cm in the control group (p = 0.001). Mean volume of bleeding during surgery was 436.6 ± 24.2 mL in the study group and 546.6 ± 30.4 mL in the control group (p = 0.004). The mean blood transfusion volume was 1.3 pints in the study group and 2.2 pints in the control group (p = 0.007) (Table 1). No significant difference in the level of pain was observed between the two groups (Fig. 1).
The preoperative serum CK levels were 86.6 ± 4.2 IU/L in the study group and 89.9 ± 3.5 IU/L in the control group (p > 0.05). On POD 1, serum CK concentration was 319.7 ± 12.1 IU/L in the study group and 582.1 ± 15.1 IU/L in the control group (p = 0.0). Serum CK concentration was 184.2 ± 9.6 IU/L in the study group and 351.1 ± 11.1 IU/L in the control group on POD 3 (p = 0.0). By POD 7, the difference disappeared. On POD 14, serum CK concentration had returned to the level before surgery (Fig. 2).
The preoperative serum aldolase level was 6.5 ± 1.2 mU/mL in the study group and 6.8 ± 0.8 mU/mL in the control group (p > 0.05). On POD 1, serum aldolase concentration was 10.1 ± 0.7 mU/mL in the study group and 18.1 ± 0.8 mU/mL in the control group (p = 0.0). This significant difference disappeared by POD 3. On POD 14, serum aldolase concentration returned to the level observed before surgery (Fig. 3).
The IL-6 level before surgery was 13.9 ± 0.3 pg/mL in the study group and 14.2 ± 0.7 pg/mL in the control group (p > 0.05). On POD 1, serum IL-6 concentration increased to 66.8 ± 2.1 pg/mL in the study group and 176.3 ± 2.5 pg/mL in the control group. On POD 3, these values were 42.9 ± 1.1 pg/mL and 105.1 ± 1.8 pg/mL, respectively (p = 0.0, p = 0.0). This significant difference disappeared by POD 7. On POD 14, IL-6 concentration returned to the preoperative level (Fig. 4).
Serum IL-8 concentration before surgery was 11.9 ± 0.4 pg/mL in the study group and 12.3 ± 0.4 pg/mL in the control group (p > 0.05). On POD 1, serum IL-8 increased to 16.4 ± 2.4 pg/mL in the study group and 22.1 ± 2.3 pg/mL in the control group (p = 0.002). On POD 3, it was 14.2 ± 2.1 pg/mL in the study group and 18.3 ± 2.0 pg/mL in the control group (p = 0.0). On POD 7, IL-8 concentrations were 14.3 ± 2.2 pg/mL in the study group and 18.2 ± 2.7 pg/mL in the control group (p < 0.05). However, this difference disappeared on POD 14 (Fig. 5).
Serum IL-10 was not different between the groups before surgery (3.2 ± 1.1 pg/mL in the study group and 3.3 ± 0.9 pg/mL in the control group). On POD 1, IL-10 level increased in both groups but that in the study group (12.2 ± 1.9 pg/mL) was significantly lower than that in the control group (21.7 ± 2.8 pg/mL; p = 0.0). On POD 3, it had decreased slightly in both groups (10.6 ± 1.8 pg/mL in the study group and 14.6 ± 2.0 pg/mL in the control group; p = 0.0). The difference disappeared by POD 7 (Fig. 6).
Serum IL-1ra concentration was not different between the groups before surgery (235.4 ± 8.9 pg/mL in the study group and 245.1 ± 7.7 pg/mL in the control group). On POD 1, it increased to 695.0 ± 10.2 pg/mL in the study group and 1,370.8 ± 16.7 pg/mL in the control group (p = 0.0). On POD 3, it decreased in both groups (543.6 ± 10.1 pg/mL in the study group and 938.3 ± 15.9 pg/mL in the control group; p = 0.0). This difference disappeared on POD 7. On POD 14, serum IL-1ra concentrations had returned to the levels seen before surgery (Fig. 7).
MI-THA has been recognized to reduce postoperative pain, lower blood loss and transfusion volumes during and after surgery, achieve an early rehabilitation, and obtain a satisfactory cosmetic outcome compared to those of C-THA. However, some reports comparing MI-THA with C-THA showed no advantages in the early treatment outcomes other than a decrease in length of the skin incision.8,9) In our study, the mean length of the skin incision in the MI-THA group (7.7 cm) was significantly shorter than that in the C-THA (14.7 cm) group and no significant difference in pain were detected after surgery. We analyzed several blood markers to clarify whether MI-THA minimizes not only the length of the skin incision but also tissue injury and systemic inflammatory reactions.
Serum CK is present in muscle in large quantities, and aldolase, an enzyme that converts fructose to glyceraldehyde and dihydroxyacetone, is also abundantly present in muscle. Serum concentrations of these enzymes increase following muscle injury which occurs due to cardiac disease, muscle disease, or trauma.10) In our study, the concentrations of serum CK and aldolase were significantly lower in the MI-THA group than those in the C-THA control group until POD 3 and 1 after surgery, respectively. This result indicates that MI-THA was more effective than C-THA for reducing the degree of muscle injury.
Early inflammatory reactions activated by pro-inflammatory cytokines are systemically normalized by anti-inflammatory cytokines responsible for suppressing the inflammatory reaction. This reaction has been called compensatory anti-inflammatory reaction syndrome (CARS). Following surgery, systemic inflammatory response syndrome is accompanied by CARS. In healthy people, an inflammatory state maintains equilibrium along a continuum between pro-inflammatory cytokines such as IL-1, tumor necrosis factor-α (TNF-α), IL-6, and IL-8 and anti-inflammatory cytokines such as IL-10, IL-13, transforming growth factor-β and IL-1ra.11,12,13,14,15,16,17) Therefore, these serologic markers properly represent the degree of tissue injury following surgery.
IL-1 and TNF-α are chemical mediators released during the earliest stages of inflammation, or the acute phase of tissue injury. Because the half-lives of IL-1 and TNF-α are approximately 6 and 20 minutes, these cytokines cannot be quantified.18) IL-6 also induces the inflammatory reaction during the early stage of tissue injury as it is secreted by macrophages and lymphocytes following a stimulus by IL-1 and TNF-α. IL-8 plays a role as a secondary mediator in the inflammatory reaction, activating neutrophils and T-cells and plays a role as a chemotactic factor that promotes migration to inflammatory sites to activate angiogenesis. The concentration of these cytokines increases with prolonged surgical time, increased blood loss, and severe tissue injury.14)
The degree of activity following tissue injury is closely associated with IL-6.13) Kim et al.15) compared cytokine levels following minimally invasive lumbar fusion and palliative lumbar fusion. According to that report, IL-6 and IL-8 were significantly lower level on POD 3 and 7, respectively, in a group undergoing minimally invasive surgery. IL-6 and IL-8 recovered to levels before surgery on POD 7 and 14, respectively. In our study, the peak concentration of serum IL-6 was 66.8 pg/mL in the study group on day 1 after surgery, which was lower than 176.3 pg/mL in the control group. The concentration decreased gradually and was significantly different between the two groups until days 3 and 7 after surgery. However, the concentration recovered to the preoperative level on POD 7. IL-8 showed a similar pattern with that of IL-6. The study group showed a peak concentration (16.4 pg/mL) on POD 1 then decreased slightly, but maintained a significant difference compared with that of the control group. On POD 14, it returned to the level before surgery. These results were similar with those of the report by Kim et al.15)
IL-10 is an anti-inflammatory cytokine secreted by T-cells, B-cells, macrophages, monocytes, and epithelial cells. It is a potent inhibitor of macrophages. IL-10 suppresses secretion and production of pro-inflammatory cytokines. It also promotes anti-inflammatory cytokines such as IL-1ra and water-soluble TNF receptor.19) IL-1ra has a direct effect on the IL-1 receptor thereby suppressing IL-1 function. It also interferes with the function of neutrophils and plays a role normalizing systemic inflammation.20) Takahashi et al.19) measured changes in serum concentrations of IL-10 and IL-1ra following vertebral fusion surgery. According to those authors, IL-10 reached a maximum level of 30.4 pg/mL immediately after surgery and returned to presurgical levels on day 7. IL-1ra increased to 1,300 pg/mL immediately after surgery and then decreased gradually. These reports show a similar pattern with our results. The serum IL-10 concentration in our study group (12.2 pg/mL) was significantly lower than that in the control group (21.7 pg/mL) on POD 1 and then decreased thereafter. Serum IL-1ra concentration in the study group (695.0 pg/mL) was also significantly lower than that in control group (1,370.8 pg/mL) on POD 1 and then decreased gradually thereafter.
Our results show that the concentrations of serum IL-6 and IL-8 were significantly lower in the MI-THA study group than those in C-THA control group on days 3 and 7 after surgery, respectively similar to the serum IL-10 and IL-1ra concentrations on POD 3. These results indicate that MI-THA could produce less of an inflammatory reaction that that of C-THA in the early recovery stage after surgery. A limitation of our study is that our results could not be applied to estimate prognosis after surgery. This deserves further study.
Our results indicate that MI-THA might be a useful surgical modality compared with C-THA based on the limited skin incision and decreased blood loss and transfusion volumes. Performing MI-THA significantly reduced tissue injury and systemic inflammation during the early stage of recovery.
Figures and Tables
Fig. 1
Visual analog pain scale (VAS) scores for both groups show no significant differences. POD: postoperative day.

Fig. 2
Serum creatine kinase (CK) concentrations for the study group show significantly lower level than the control group on postoperative day (POD) 1 (p = 0.0) and 3 (p = 0.0).

Fig. 3
Serum aldolase concentrations for the study group show significantly lower level than the control group on postoperative day (POD) 1 (p = 0.0).

Fig. 4
Serum interleukin (IL)-6 concentrations for the study group show significantly lower level than the control group on postoperative day (POD) 1 (p = 0.0) and 3 (p = 0.0).

Fig. 5
Serum interleukin (IL)-8 concentrations for the study group show significantly lower level than the control group on postoperative day (POD) 1 (p = 0.002), 3 (p = 0.0), and 7 (p = 0.0).

Fig. 6
Serum interleukin (IL)-10 concentrations for the study group show significantly lower level than the control group on postoperative day (POD) 1 (p = 0.0) and 3 (p = 0.0).

References
1. Berger RA. Mini-incisions: two for the price of one! Orthopedics. 2002; 25(5):472. 498.
2. Hartzband MA. Posterolateral minimal incision for total hip replacement: technique and early results. Orthop Clin North Am. 2004; 35(2):119–129.
3. Sculco TP, Jordan LC, Walter WL. Minimally invasive total hip arthroplasty: the Hospital for Special Surgery experience. Orthop Clin North Am. 2004; 35(2):137–142.
4. Soni RK. An anterolateral approach to the hip joint. Acta Orthop Scand. 1997; 68(5):490–494.
5. Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics. 2002; 25(10):1031–1043.
6. Wright JM, Crockett HC, Delgado S, Lyman S, Madsen M, Sculco TP. Mini-incision for total hip arthroplasty: a prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004; 19(5):538–545.
7. Marcy GH, Fletcher RS. Modification of the posterolateral approach to the hip for insertion of femoral-head prosthesis. J Bone Joint Surg Am. 1954; 36(1):142–143.
8. Cameron HU. Mini-incisions: visualization is key. Orthopedics. 2002; 25(5):473.
9. Ogonda L, Wilson R, Archbold P, et al. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes: a prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005; 87(4):701–710.
10. Dufour DR, Lott JA, Henry JB. Clinical enzymology. In : Henry JB, editor. Clinical diagnosis and management by laboratory methods. 20th ed. Philadelphia, PA: WB Saunders;2001. p. 281–303.
11. Parsons PE. Interleukin-10: the ambiguity in sepsis continues. Crit Care Med. 1998; 26(5):818–819.
12. Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996; 224(5):647–664.
13. Rossi GA. COPD patients or "healthy smokers": is IL-8 synthesis and release the borderline? Respiration. 2003; 70(5):457–459.
14. Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994; 6(2):181–186.
15. Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine (Phila Pa 1976). 2006; 31(6):712–716.
16. Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996; 24(7):1125–1128.
17. Igonin AA, Armstrong VW, Shipkova M, Lazareva NB, Kukes VG, Oellerich M. Circulating cytokines as markers of systemic inflammatory response in severe community-acquired pneumonia. Clin Biochem. 2004; 37(3):204–209.
18. Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000; 127(2):117–126.
19. Takahashi J, Ebara S, Kamimura M, et al. Pro-inflammatory and anti-inflammatory cytokine increases after spinal instrumentation surgery. J Spinal Disord Tech. 2002; 15(4):294–300.
20. Barnes PJ. IL-10: a key regulator of allergic disease. Clin Exp Allergy. 2001; 31(5):667–669.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download