Abstract
Background
Within the lateral pillar classification of the Legg-Calvé-Perthes (LCP) disease, hips seem quite variable in the pattern of fragmentation as seen in radiographs. The purpose of this study was to determine: if it is possible to reliably subdivide the lateral pillar groups into femoral head fragmentation patterns, and if such a subdivision of the lateral pillar groupings is clinically useful in managing LCP disease.
Methods
Two hundred and ninety-three anteroposterior radiographs taken at the maximal fragmentation stage (189 lateral pillar B, 57 B/C border, and 47 C hips; mean bone/chronologic age at the time of first visit, 6.2/7.9 years) and at skeletal maturity (mean age, 16.6 years) were analyzed. We distinguished 3 fragmentation patterns in each pillar group based on the region of major involvement. We tested the inter- and intraobserver reliability of our classification system and analyzed the relationships between the fragmentation patterns and the Stulberg outcomes as well as other factors such as surgical treatment and age.
Results
Inter- and intraobserver consistency in fragmentation pattern assignments was found to be substantial to excellent. A statistically significant trend (p = 0.001) in the proportion of Stulberg III or IV outcomes in comparison with Stulberg I and II was only found for the different fragmentation patterns in our lateral pillar B patients: fragmentation patterns having mainly lateral-central necrosis led to poor outcomes. No significant association was found between fragmentation patterns and Stulberg outcomes in pillar groups B/C border and C.
In Legg-Calvé-Perthes (LCP) disease, Catterall1) and lateral pillar2,3) classifications have been widely used in clinical practice to assess patients at their fragmentation stage. However, several authors4,5,6) have found that 31%-45% of hips may change their lateral pillar classification during the course of treatment; thus an evaluation based on the initial radiographic findings may not be correlated with final outcomes in these patients. Moreover, the fragmentation patterns of the capital femoral epiphysis are quite variable, leading some physicians to suspect that certain patterns of fragmentation are likely to have satisfactory final outcomes while others (in the same lateral pillar class) often lead to unsatisfactory outcomes even in younger patients. Although many authors3,6,7,8,9,10) have found better interobserver agreement with the lateral pillar classification than with the Catterall classification, little attention has been given to the pattern of fragmentation, which varies with the location and the amount of necrosis of the epiphysis.
We hypothesized that the pattern of fragmentation might correlate with the radiographic outcome at skeletal maturity and developed a simple classification system of fragmentation patterns in LCP disease based on the location and the amount of necrosis of the epiphysis. The purpose of this study was to determine: (1) if it is possible to reliably subdivide the lateral pillar groups into femoral head fragmentation patterns, and (2) if such a subdivision of the lateral pillar groupings is clinically useful in managing LCP disease.
This study was designed as a retrospective review of data from a controlled, long-term multicenter study and was therefore exempted from an approval by the Institutional Review Board of Pusan National University Hospital.
All patients were older than six years of age at the onset of symptoms and had no prior treatment. Patients with prior steroid treatment or other systemic or local disorders associated with the hip were excluded. Hips that had reached the stage of early reossification were also excluded. Anteroposterior (AP) and frog-leg lateral radiographs were collected at four, eight, and twelve months from the onset of symptoms during the first year and at least yearly thereafter. Radiographic changes necessary for lateral pillar classification were usually evident within 6 months after the onset of the symptoms.
We used AP radiographs of the pelvis made in the maximal fragmentation stage of the disease to assign the fragmentation pattern. We often used several radiographs taken several months apart in the fragmentation stage to compensate for differences in radiographic quality as well as to accommodate for changes due to progression of the fragmentation.
The data from the multicenter study consisted of 346 hips: 223 pillar B, 63 B/C border, and 60 pillar C. However, due to the poor quality of some of the original radiographs and the fact that our classification could not be applied to few hips, our final hip numbers were as follows: 189 pillar B, 57 B/C border, and 47 pillar C (total, 293 hips). All of these patients had reached skeletal maturity (mean age, 16.6 years; range, 11.3 to 23.1 years). The mean bone age was 6.2 years (range, 3.0 to 13.0 years) and the chronologic age at the time of induction was 7.9 years (range, 6.0 to 12.0 years). Mean bone and chronologic ages for each pillar group were: B, 6.4 and 8.0; B/C border, 6.2 and 8.0; C, 5.6 and 7.5. Skeletal age was determined from radiographs of the hand and wrist with use of the Greulich and Pyle atlas.11) Skeletal maturity means that all the carpals, metacarpals and phalanges are completely developed and that their physes are closed. Epiphyseal fusion of the ulna and radius was also included in skeletal maturity. When no radiograph of the wrist was available, the skeletal age was determined from the pelvic radiograph by the Oxford bone age method;12) the triradiate cartilage and the greater trochanter are closed and the iliac apophysis shows Risser IV or V at skeletal maturity.
Of 190 non-surgically treated patients, the maximal fragmentation stage was identified at the time of the first visit to hospital in 11 patients; in 53 patients during the 6 months following the first visit; in 58 patients 6-12 months after the first visit; in 40 patients 12-18 months after the first visit; in 13 patients 18-24 months after the first visit; and in 15 patients at least 2 years after the first visit. Of 103 surgically treated patients, the maximal fragmentation stage was identified at the time of first visit to hospital in 6 patients; in 42 patients during the first 6 months; in 30 patients after 6-12 months; in 11 patients after 12-18 months; in 6 patients after 18-24 months; and in 9 patients at least 2 years after the first visit. Thirty-nine patients showed the maximal fragmentation stage before surgical treatment and 64 patients (62.1%) showed it after surgery.
Our 189 pillar B hips were managed as follows: 61 brace, 40 Salter osteotomy, 32 femoral osteotomy, 48 range of motion (ROM) exercise, and 8 patients received symptomatic treatment only. Of 57 pillar B/C border hips received 19 braces, 18 Salter osteotomy, 6 femoral osteotomy, 11 ROM exercise, and 3 other treatments (symptomatic treatment). Of 47 pillar C hips received 24 braces, 3 Salter osteotomy, 4 femoral osteotomy, 11 ROM exercise, and 5 other treatments (symptomatic treatment).
We distinguished 3 fragmentation patterns in each pillar group as follows: (1) Lateral pillar B (Fig. 1): pattern 1, necrosis primarily central; pattern 2, necrosis medial and central; pattern 3, necrosis lateral and central. (2) Lateral pillar B/C border: pattern 1, a very narrow pillar (2-3 mm wide) with > 50% of the original height; pattern 2, a lateral pillar with exactly 50% of the original height depressed relative to the central pillar (as described by Herring et al.2)); pattern 3, a lateral pillar with very little ossification but with at least 50% of the original height. (3) Lateral pillar C (Fig. 2): pattern 1, ossific nucleus evenly flattened; pattern 2, necrosis mainly lateral and central; pattern 3, entire ossific nucleus severely fragmented. Our basic concept in creating the fragmentation patterns was the extent of involvement of the epiphysis and the severity of involvement in the lateral pillar. The fragmentation pattern 1 was the least and pattern 3 the most affected in all 3 pillar groups. In lateral pillar B, fragmentation pattern 3 presented a mainly lateral involvement. In B/C border, lucency was evident in the lateral pillar in pattern 3, with more involvement of the middle column than in patterns 1 and 2. A severe involvement of the whole epiphysis with more metaphyseal changes was typical in pattern 3 of pillar C.
The classification of fragmentation patterns was done by 3 observers (1 staff orthopedist, 1 senior resident, and 1 junior resident) in 3 trials. Each observer took at least 2 hours break between the trials as the number of hips to classify was high (239) and the likelihood of fatigue was great. All observers were instructed by the senior author in a ten-minute lecture on the details of the fragmentation classification system using representative drawings of each of the fragmentation patterns. Each observer was given his own copy of the 9 drawings to use while assigning fragmentation patterns. Each observer viewed the digitalized radiographs on his own 21-inch monitor (SyncMaster Magic CX21OT, Samsung, Suwon, Korea). The fragmentation patterns were written down by the observers without discussion.
The observers got the radiographs presorted into pillar groups as we were testing the reliability of our fragmentation pattern classification and not the pillar classification. Using 3 observers and 3 trials, we had 9 assignments for each radiograph. For the final accepted fragmentation pattern assignment of each film (necessary for the comparison with Stulberg outcome), we simply took the majority view: this comprised at least 5 of the 9 assignments in all cases. Of the 293 radiographs provided us each with 3 fragments with 1 from each observer.
Weighted kappa statistics were used to measure inter- and intrarater reliability. We calculated weighted kappa statistics and 95% confidence intervals for the kappa statistic using Stata ver. 11.1 (Stata Co., College Station, TX, USA). A kappa value of 1.00 meant perfect agreement, whereas 0.00 meant agreement no better than chance. A kappa value of 0.00 to 0.20 indicates slight agreement; 0.21 to 0.40 is considered to be fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and 0.81 to 1.0, indicates excellent agreement according to Landis and Koch.13) The Mantel-Haenszel chi-square test was used to analyze if the different fragmentation patterns correlate with different Stulberg outcome14) rates. Ordinal multivariable logistic regression was used to analyze the association of fragmentation pattern, surgical treatment and age with Stulberg outcome. Statistical analyses were performed using SPSS ver. 12 (SPSS Inc., Chicago, IL, USA).
Pattern 1 was the most common fragmentation pattern of our pillar B patients while pattern 2 was the most numerous for both, B/C border and C (Table 1).
Table 2 shows the mean kappa values (with 95% confidence intervals) by pillar group for inter- and intraobserver reliability of our classification system. The mean values for interobserver reliability are all quite high, ranging from 0.721 to 0.825. The mean values for intraobserver reliability fell in the same range; these values imply substantial to excellent agreement.
A statistically significant trend (p = 0.001) was found in the proportion of Stulberg III or IV outcomes in comparison with Stulberg I and II for the different fragmentation patterns in our pillar B patients (Table 3). No significant association was found between fragmentation patterns and Stulberg outcomes in pillar groups B/C border and C.
If we used fragmentation pattern 1 as the reference group, a hip with fragmentation pattern 3 was found to be 3.48 times more likely to result in a Stulberg III or IV outcome in pillar B patients (p < 0.001) (Table 4); however, fragmentation pattern 2 hips did not have a much greater likelihood of a Stulberg III or IV outcome compared to pattern 1. Also, a patient aged at least 8 years showed a 3.66 times more likelihood to have a Stulberg III or IV result than a patient aged under 8 years (p < 0.001). A patient aged at least 8 years could expect an even greater increase of likelihood (6.39 times) of a poor Stulberg outcome than a younger patient in pillar B/C border patients (p = 0.002). No statistically significant relationship among fragmentation pattern, treatment and age was found in the pillar C group.
Although fragmentation of the ossific nucleus is one of the most characteristic features of LCP disease, the exact pattern of fragmentation depends on the complex and dynamic interplay of a range of factors. Consequently we might expect that any relationship between fragmentation pattern and outcome would be nebulous at best.
The multivariable logistic regression model used in this study offers certain advantages in a disorder such as in LCP disease, where many different factors simultaneously influence the final outcome. Allowing us to analyze the role of each factor, taking into account the effects of the other factors is one of it. The influence of fragmentation pattern on the final Stulberg outcome was expressed using the odds ratio15) while adjusting for treatment pattern and age. The odds ratio gives an estimate of relative measure of risk of a particular outcome as compared to some baseline levels of risk, such as having fragmentation pattern 1. Another measure is that it explicitly considers the interaction terms of the studied variables.
Overall, patients with lateral pillar B hips with fragmentation pattern 3 (lateral-central necrosis) showed significantly worse results than patients with fragmentation pattern 1 (central necrosis). However, this was not found in pillar B/C border and C hips. Patient's age higher than 8 years was also a significant contributor to poor results in our pillar B and B/C border groups. We carefully looked not only at the single variables age, treatment pattern and fragmentation pattern, but also at the interaction terms among these variables for each pillar group in the light of the possibility that the effectiveness of surgery might depend upon the age of the patient. The beneficial effect of surgical treatment was significant or nearly so in patients with pillar B and B/C border hips, which was confirmed by the low likelihood of a poor Stulberg outcome. The lack of significant effects of any of the variables in the patients with pillar C hips was not surprising since the interaction of surgical treatment and age with Stulberg outcome was not clearly demonstrated in the larger multicenter study.14)
This study has several limitations: (1) Our sample size for pillar B/C border and C patients is small. Theoretically it would be desirable to analyze the fragmentation pattern three-dimensionally. We began this study with both AP and frog-leg lateral radiographs, but this resulted in smaller subgroups, each of which contained of too few patients for statistical analysis. As the lateral pillar classification uses the AP radiograph only, we also opted for a simplistic approach using AP radiographs. The fragmentation pattern on lateral radiographs should also be included if possible for a more refined analysis. (2) Lateral pillar assignments were included with the multi-center study data2,14) and were not tested in this study. If we had not presorted the images into pillar groups, but rather had allowed the observers free choice among 9 fragmentation patterns, it would have been more difficult to evaluate our classification of fragmentation patterns alone. (3) Our fragmentation patterns were determined at maximal fragmentation. We selected the radiographs showing maximal fragmentation from a series of radiographs of each patient and a considerable number of patients showed maximal fragmentation after surgical treatment. We believe treatment should begin after the lateral pillar group is determined, but before there is substantial deformity of the femoral head. The lateral pillar group can normally be determined at an average of 6 or 7 months after disease onset.2,5) The authors cannot say whether the surgery actually altered the lateral pillar grouping of the hip, but we certainly believe that surgery is beneficial for remodeling of the hip. Therefore we can only suggest that the fragmentation pattern might be helpful in estimating the future prognosis when it is discerned as early as possible.
In conclusion, this study confirms that: (1) At least for pillar B, if the main area of fragmentation is located laterally, it leads to a worse Stulberg outcome as it is already told by the lateral pillar group.3,14) (2) Given that the pattern of fragmentation in lateral pillar B/C border and C patients seems to add little of predictive value, and especially since the maximal fragmentation stage may occur quite late in the disease process, our hypothesis appears to be more of theoretical interest than of clinical usefulness in the management of LCP disease. A finer subdivision of the lateral pillar classification was not clinically useful in the management of LCP disease.
Figures and Tables
 | Fig. 1Fragmentation patterns (and examples) in a lateral pillar B Legg-Calvé-Perthes disease. (A) Pattern 1, necrosis primarily central. (B) Pattern 2, necrosis medial and central. (C) Pattern 3, necrosis lateral and central. |
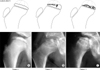 | Fig. 2Fragmentation patterns (and examples) in a lateral pillar C Legg-Calvé-Perthes disease. (A) Pattern 1, ossific nucleus evenly flattened. (B) Pattern 2, necrosis mainly lateral. (C) Pattern 3, entire ossific nucleus fragmented. |
References
1. Catterall A. The natural history of Perthes' disease. J Bone Joint Surg Br. 1971; 53(1):37–53.
2. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am. 2004; 86(10):2103–2120.
3. Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992; 12(2):143–150.
4. Kuroda T, Mitani S, Sugimoto Y, et al. Changes in the lateral pillar classification in Perthes' disease. J Pediatr Orthop B. 2009; 18(3):116–119.
5. Lappin K, Kealey D, Cosgrove A. Herring classification: how useful is the initial radiograph? J Pediatr Orthop. 2002; 22(4):479–482.
6. Park MS, Chung CY, Lee KM, Kim TW, Sung KH. Reliability and stability of three common classifications for Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 2012; 470(9):2376–2382.
7. Nathan Sambandam S, Gul A, Shankar R, Goni V. Reliability of radiological classifications used in Legg-Calve-Perthes disease. J Pediatr Orthop B. 2006; 15(4):267–270.
8. Podeszwa DA, Stanitski CL, Stanitski DF, Woo R, Mendelow MJ. The effect of pediatric orthopaedic experience on interobserver and intraobserver reliability of the herring lateral pillar classification of Perthes disease. J Pediatr Orthop. 2000; 20(5):562–565.
9. Ritterbusch JF, Shantharam SS, Gelinas C. Comparison of lateral pillar classification and Catterall classification of Legg-Calvé-Perthes' disease. J Pediatr Orthop. 1993; 13(2):200–202.
10. Simmons ED, Graham HK, Szalai JP. Interobserver variability in grading Perthes' disease. J Bone Joint Surg Br. 1990; 72(2):202–204.
11. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press;1959.
12. Acheson RM. The Oxford method of assessing skeletal maturity. Clin Orthop. 1957; 10:19–39.
13. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159–174.
14. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004; 86(10):2121–2134.
15. Bland JM, Altman DG. Statistics notes: the odds ratio. BMJ. 2000; 320(7247):1468.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download