Randomized controlled trials (RCT) are known as the best method to prove causality in spite of various limitations. Random allocation is a technique that chooses individuals for treatment groups and control groups entirely by chance with no regard to the will of researchers or patients' condition and preference. This allows researchers to control all known and unknown factors that may affect results in treatment groups and control groups.
Allocation concealment is a technique used to prevent selection bias by concealing the allocation sequence from those assigning participants to intervention groups, until the moment of assignment. Allocation concealment prevents researchers from influencing which participants are assigned to a given intervention group. This process must be included in the experiment for the success of any RCT.
Blinding refers to keeping trial participants, health care providers, assessors or data collectors unaware of the assigned intervention, so that they will not be influenced by that knowledge. This process is conducted to minimize possible bias in implementation, dropouts, measurements, etc. Blinding is not always feasible for RCT but should be implemented if possible.
Randomization, allocation concealment and blinding should be well implemented and should be described in the paper.
On the other hand, many researchers are still unfamiliar with how to do randomization, and it has been shown that there are problems in many studies with the accurate performance of the randomization and that some studies are reporting incorrect results. So, we will introduce the recommended way of using statistical methods for a randomized controlled study and show how to report the results properly.
The easiest method is simple randomization. If you assign subjects into two groups A and B, you assign subjects to each group purely randomly for every assignment. Even though this is the most basic way, if the total number of samples is small, sample numbers are likely to be assigned unequally. For this reason, we recommend you to use this method when the total number of samples is more than 100.
We can create a block to assign sample numbers equally to each group and assign the block.
If we specify two in one block (the so-called block size is two), we can make two possible sequences of AB and BA. When we randomize them, the same sample numbers can be assigned to each group. If the block size is four, we can make six possible sequences; these are AABB, ABAB, ABBA, BAAB, BABA, BBAA, and we randomize them.
However, there is a disadvantage in that the executer can predict the next assignment. We can easily know the fact that B comes after A if the block size is two and if the block size is four; we can predict what every 4th sample is. This is discordant with the principle of randomization. To solve this problem, the allocator must hide the block size from the executer and use randomly mixed block sizes. For example, the block size can be two, four, and six.
Randomization is important because it is almost the only way to assign all the other variables equally except for the factor (A and B) in which we are interested. However, some very important confounding variables can often be assigned unequally to the two groups. This possibility increases when the number of samples is smaller, and we can stratify the variables and assign the two groups equally in this case.
For example, if the smoking status is very important, what will you do? First, we have two methods of randomization that we learned previously. There are two randomly assigned separate sequences for smokers and non-smokers. Smokers are assigned to the smoker's sequences, and non-smokers are assigned to the non-smoker's sequences. Therefore, both smokers and non-smokers groups will be placed equally with the same numbers.
So we can use 'simple randomization with/without stratification' or 'block randomization with/without stratification.' However, if there are multiple stratified variables, it is difficult to place samples in both groups equally with the same numbers. Usually two or fewer stratified variables are recommended.
Although there are websites or common programs for randomization, let us use an Excel file. Download the attached file in http://cafe.naver.com/easy2know/6427. It is in a 'Read-only' state, but there is no limit in function; it is in the 'Read-only' state only to prevent accidental modification.
Due to the nature of Excel, if there is a change, it creates a new random number accordingly. If we input any number instead of '2' in the orange-colored cell and click the 'enter key,' it creates new random sequences (Fig. 1). The sequences are the result of simple randomization. The numbers in the right column show the numbers of the total sample. Basically the numbers are up to 1,000, but if you need to, you can extend the numbers with the AutoFill function in Excel.
Fig. 2 shows an example of randomization when the block size is four. Also, there are numbers of the total samples in the right column.
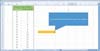
Fig. 3 shows an example of block randomization when the block size is two and four. Total eight kinds of blocks inside of the red-dotted line are assigned at random. The left column is for allocation and the right column is for the total sample size.
By the way, www.randomization.com can do block randomization for up to four kinds of block sizes and it is very easy to perform as well. Fig. 4 shows the general features and an example.
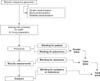
How to implement these techniques can vary by each trial. The following is only one of the examples of how these can be implemented in real trial. You may change the details of the example for your experiment. Figures of randomization and allocation concealment can also be adjusted to your needs (Fig. 5).
An independent researcher makes random allocation cards using computer-generated random numbers. He keeps the original random allocation sequences in an inaccessible third place and works with a copy. Since the executers can get confused with the original coding of A and B later, the allocator should record exactly what these codes mean to avoid further confusion.
When the purpose of the study is a surgical procedure, instead of using A and B, different names that distinguish exactly between the surgical procedures should be used (for example, 'the anterior approach' and 'the posterior approach'). It is convenient to reproduce the contents of the Excel file to a Word file, and enlarge the text font after replacing A with 'the anterior approach' (page break) and B with 'the posterior approach' (page break). Next, you print it out and put each of the sheets one by one into each envelope (Fig. 6).
Here in Fig. 6, '^m' is a special character for manual page break. After setting it as shown, you click 'all change' and print it out. Then we can get it printed per sheet. The inside of the envelope should not be visible from the outside, and it has to be printed out for each one and put in an envelope after being folded several times. In some papers, even aluminum foil was used to hide the print to prevent it from being read with a flash of light.
There are serial numbers on the outside of the envelopes. Input date, time, patient ID, results after the procedure, etc. usually will be recorded on the envelope or another sheet inside of the envelope, also.
An independent nurse (researcher) prepares syringes with "drug A" and "drug B" and puts them into envelopes according to the allocation orders. These syringes cannot be distinguished because they contain the same colored liquid with the same volume. Or pills or tablets with the same color and shape (placebo) will be put into the envelopes according to the allocation orders.
In the case of surgical treatment, an independent researcher prepares the envelopes, including writing the treatment name on a sheet of paper inside it. In the operation room, another independent nurse (researcher) opens the envelope and informs the doctor to do the treatment that is written on the paper in the envelope.
Another independent nurse injects the drug or the doctor performs the operation according to the order. The patient's ID, date, time and other information are recorded on each envelope. The nurse and the patient would not know what drugs are injected (double blinded). The doctor knows the treatment and the patient does not know it (one blinded). The preparer retrieves the envelopes and checks to see if the operation (and injection) was done as planned.
In the case of broken or lost syringes, the preparer figures out what the number of the envelope it is and replaces the envelope with the same drug according to the allocation.
The envelopes should be opened just before the injection or operation. For example, when a patient comes, an envelope is opened; however, if this does not meet the criteria for the performance of the study, this can be cancelled. Also, if the operator finds out before an operation the tool that is to be inserted, it is impossible to get the operation as planned. For example, even though plate A was assigned to be used, if the patient was indicated to have some other surgery because of infection or severe osteoporosis, you will waste an envelope and it will cause confusion as well as violate the randomization. All these cases should be mentioned as inclusion criteria and exclusion criteria in advance. To avoid this, the envelopes should be opened just before the operation or injection if possible.
However, in cases where the operation tool is so big that two tools cannot be prepared at the same time, or the preparation takes a lot of money (robotic surgery, etc.) or time (liver transplantation, etc.), the envelopes can be opened in advance.
Also, although you open an envelope and choose the procedure that you see, other conditions that affect the outcome can occur. For example, the patient could be admitted to the intensive care unit for medical problems after treatment, or may not get enough rehabilitation treatment for some other reasons.
In this case, it is an important issue whether to consider this as a follow-up loss or exclude this case from the study. We can deal with this issue by focusing on intention-to-treat analysis and per-protocol analysis. We will study this later when we get a chance.
From 1988 to 2000, 72 of 2,468 papers (2.9%) in the Journal of Born and Joint Surgery were RCTs.1) It has been suggested that in some of the papers, randomization was not completely done or the result was not properly reported. According to the analysis of RCTs using painkillers from the January issue in 1966 to the June issue in 2006, 23.9% of the papers were inadequate in terms of the randomization.2) It would be helpful to see a CONSORT checklist and examples. The following were used in the actual papers and extracted from examples in the CONSORT (http://www.consort-statement.org).
"Independent pharmacists dispensed either active or placebo inhalers according to a computer generated randomization list."
"For allocation of the participants, a computer-generated list of random numbers was used."
"Randomization sequence was created using Stata 9.0 (StataCorp, College Station, TX, USA) statistical software and was stratified by center with a 1:1 allocation using random block sizes of 2, 4, and 6."
"Participants were randomly assigned following simple randomization procedures (computerized random numbers) to 1 of 2 treatment groups."
We can apply the above examples to our case as follows: Randomization sequence was created using Excel 2007 (Microsoft, Redmond, WA, USA) with a 1:1 allocation using random block sizes of 2 and 4 by an independent doctor. In this way, sequence generation and type of randomization can be expressed at the same time.
"The doxycycline and placebo were in capsule form and identical in appearance. They were pre-packed in bottles and consecutively numbered for each woman according to the randomization schedule. Each woman was assigned an order number and received the capsules in the corresponding pre-packed bottle."
"The allocation sequence was concealed from the researcher (JR) enrolling and assessing participants in sequentially numbered, opaque, sealed and stapled envelopes. Aluminum foil inside the envelope was used to render the envelope impermeable to intense light. To prevent subversion of the allocation sequence, the name and date of birth of the participant was written on the envelope and a video tape made of the sealed envelope with participant details visible. Carbon paper inside the envelope transferred the information onto the allocation card inside the envelope and a second researcher (CC) later viewed video tapes to ensure envelopes were still sealed when participants' names were written on them. Corresponding envelopes were opened only after the enrolled participants completed all baseline assessments and it was time to allocate the intervention."
The second example was described in great detail, and we can guess how important the randomization and concealment were.
"Determination of whether a patient would be treated by streptomycin and bed-rest (S case) or by bed-rest alone (C case) was made by reference to a statistical series based on random sampling numbers drawn up for each sex at each center by Professor Bradford Hill (this means that the stratification was done by sex and center); the details of the series were unknown to any of the investigators or to the coordinator. After acceptance of a patient by the panel, and before admission to the streptomycin center, the appropriate numbered envelope was opened at the central office; the card inside told, if the patient was to be an S or a C case, and this information was then given to the medical officer of the center."
"Details of the allocated group were given on colored cards contained in sequentially numbered, opaque, sealed envelopes. These were prepared at the NPEU and kept in an agreed location on each ward. Randomization took place at the end of the 2nd stage of labor when the midwife considered a vaginal birth was imminent. To enter a woman into the study, the midwife opened the next consecutively numbered envelope."
"Block randomization was by a computer generated random number list prepared by an investigator with no clinical involvement in the trial. We stratified by admission for an oncology related procedure. After the research nurse had obtained the patient's consent, she telephoned a contact who was independent of the recruitment process for allocation consignment."
"Whereas patients and physicians allocated to the intervention group were aware of the allocated arm, outcome assessors and data analysts were kept blinded to the allocation."
"Blinding and equipoise were strictly maintained by emphasizing to intervention staff and participants that each diet adheres to healthy principles, and each of them is advocated by certain experts to be superior for long-term weight-loss. Except for the interventionists (dieticians and behavioral psychologists), investigators and staff were kept blind to diet assignment of the participants. The trial adhered to established procedures to maintain separation between staff that take outcome measurements and staff that deliver the intervention. Staffs who obtained outcome measurements were not informed of the diet group assignment. Intervention staffs, dieticians and behavioral psychologists who delivered the intervention did not take outcome measurements. All investigators, staffs, and participants were kept masked to outcome measurements and trial results."
In short, in a paper, we have to report who was kept blinded. In the case of physical therapy or surgery, keeping the surgeon blinded would be difficult or even impossible; however, blinding is possible for the person who measures the outcome. Anyhow, all individuals who were kept blinded must be described in the report.
To help with all the procedures of a fully qualified RCT, the following systems including electronic case report forms (eCRFs) are available for researchers.
iCReaT (clinical research and trial management system) in Korea Centers for Disease Control & Prevention (KCDC; http://icreat.nih.go.kr): free for pre-educated and qualified researchers; there are regular education programs once a month, and some hospitals (for example, Severance Hospital) have their own educational programs. An English version will be available soon for non-Korean researchers.
MRCC (https://mrcc.snuh.org): for Seoul National University Hospital only. It is relatively inexpensive and includes statistical counseling.
Velos (http://eresearch.ncc.re.kr): a world-famous system and very expensive; it is available at National Cancer Center in Korea (http://ncc.re.kr/crcc/).
eCRFs are very convenient as well as helpful to improve the quality of research and their advantages are summarized in the table (Table 1).
Figures and Tables
 | Fig. 3Block randomization when the block size is two and four. Total eight blocks in the red-dotted line are assigned at random. The left column is for allocation and the right column is for the total sample size. |
 | Fig. 4
www.randomization.com can do block randomization more easily. In this figure, the block size is 2, 4, and 6 when the total samples are 88. |
References
1. Bhandari M, Richards RR, Sprague S, Schemitsch EH. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. J Bone Joint Surg Am. 2002; 84(3):388–396.
2. Montané E, Vallano A, Vidal X, Aguilera C, Laporte JR. Reporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic review. BMC Clin Pharmacol. 2010; 10:2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download