Abstract
Background
Anterior interbody fusion has previously been demonstrated to increase neuroforaminal height in a cadaveric model using cages. No prior study has prospectively assessed the relative change in magnetic resonance imaging (MRI) demonstrated neuroforaminal dimensions at the index and supradjacent levels, after anterior interbody fusion with a corticocancellous allograft in a series of patients without posterior decompression. The objective of this study was to determine how much foraminal dimension can be increased with indirect foraminal decompression alone via anterior interbody fusion, and to determine the effect of anterior lumbar interbody fusion on the dimensions of the supradjacent neuroforamina.
Methods
A prospective study comparing pre- and postoperative neuroforaminal dimensions on MRI scan among 26 consecutive patients undergoing anterior lumbar interbody fusion without posterior decompression was performed. We studies 26 consecutive patients (50 index levels) that had undergone anterior interbody fusion followed by posterior pedicle screw fixation without distraction or foraminotomy. We used preoperative and postoperative MRI imaging to assess the foraminal dimensions at each operated level on which the lumbar spine had been operated. The relative indirect foraminal decompression achieved was calculated. The foraminal dimension of the 26 supradjacent untreated levels was measured pre- and postoperatively to serve as a control and to determine any effects after anterior interbody fusion.
Results
In this study, 8 patients underwent 1 level fusion (L5-S1), 12 patients had 2 levels (L4-S1) and 6 patients had 3 levels (L3-S1). The average increase in foraminal dimension was 43.3% (p < 0.05)-19.2% for L3-4, 57.1% for L4-5, and 40.1% for L5-S1. Mean pre- and postoperative supradjacent neuroforaminal dimension measurements were 125.84 mm2 and 124.89 mm2, respectively. No significant difference was noted (p > 0.05).
Conclusions
Anterior interbody fusion with a coriticocancellous allograft can significantly increase neuroforaminal dimension even in the absence of formal posterior distraction or foraminotomy; anterior interbody fusion with a coriticocancellous allograft has little effect on supradjacent neuroforaminal dimensions.
Radiculopathy caused by degenerative disc disease is one of the most common indications for spine surgery. Treatment options include direct methods of nerve root decompression (e.g., foraminotomy) as well as indirect methods of decompression (e.g., distraction across the segment). Such indirect methods are commonly employed within the cervical spine.1-3) In contrast, radiculopathy arising from lumbar foraminal stenosis is generally addressed through more direct posteriorly-based techniques.4) Possible reasons for this difference may include the relative safety of the posterior lumbar approach, as well as uncertainty as to the degree of decompression attainable through indirect methods of distraction such as anterior lumbar interbody fusion (ALIF).
Relatively few authors have sought to determine how much indirect decompression of the lumbar nerve root can be achieved through anterior distraction alone. In addition, little has been written about the effects of lumbar fusion and segmental distraction upon the dimensions of the supradjacent neuroforamina. If the neural foramen could be reliably expanded through indirect means without negative effect on the supradjacent segment foramen dimension, it is possible that the indications for certain current and future anterior lumbar procedures could be expanded.
To our knowledge, no prior study has assessed prospectively the relative change in magnetic resonance imaging (MRI) demonstrated neuroforaminal dimensions in a consecutive series of patients after anterior interbody fusion without any posterior decompression. We sought to compare preoperative foraminal dimensions at each operative level with the corresponding postoperative size to determine the change attributable to anterior distraction alone in patients undergoing surgery for degenerative disease of the lumbar spine.
Institutional Review Board approval was obtained for the enrollment and comparison of pre- and postoperative MR imaging for patients included in this investigation. Thirty consecutive patients scheduled for anterior interbody fusion followed by posterior pedicle screw fixation without distraction or foraminotomy were consented and enrolled into the study. No compression or distraction force was applied definitely.
Preoperative and postoperative MRI was used to assess the foraminal dimensions at each level on which the lumbar spine has been operated and the relative indirect foraminal decompression achieved. In addition, the foraminal dimension of the supradjacent untreated level (i.e., the level immediately cephalad to the most proximal fused segment) was measured pre- and postoperatively to serve as a control and to find any effect after anterior interbody fusion. All measurements were performed by spine surgeons under the supervision of a neuroradiologist.
Of the 30 consecutive enrollees, 26 patients (50 index levels and 26 supradjacent levels) were included within the study. Four patients were excluded because the pre- and postoperative saggital MRI slices through the neural foramina were not considered comparable. For each included patient, the neural foramina on a single side (i.e., either left or right) were chosen for comparison between pre- and postoperative images. Sides were chosen based on which saggital slices corresponded most closely between the preoperative and postoperative image series. For the index levels, the right side was best visualized for 28 levels, and the left side for 22 levels. For the supradjacent levels, for 11 patients, the right side sagittal images most closely corresponded and were used for pre- and postoperative comparison. For the remaining 15 patients, the left sided sagittal images most closely corresponded and were the basis for pre- and postoperative comparison.
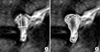
If only printed MRI images were available, the foramen was modeled as an ellipse with foraminal dimension = (anteroposterior [AP] diameter/2) × (transverse diameter/2) × Π. If software measurement was possible, the foraminal dimension was measured with Vitrea 2 (ver. 3.9, VITAL, Minnetonka, MN, USA). For 12 patients (70 levels including supradjacent segment) for whom both printed and digital images were available, foraminal dimension was calculated and compared using both methods. Pearson's correlation coefficient was 0.998, and the elliptical model was considered reliable and validated (Fig. 1).
At each level studied, the procedure performed was an anterior interbody fusion and posterior pedicle screw fixation without posterior distraction or posterior foraminotomy. Eight fellowship trained spine surgeons at a single institution participated. At each level, a corticocancellous allograft (Precision, Medtronic Sofamor Danek, Memphis, TN, USA) was placed in the disk space after thorough anterior diskectomy. Each surgeon independently chose the most appropriate graft height for each level based upon clinical experience and judgment.
Postoperative MRIs were performed on all patients within 2 to 5 days of surgery. Working in concert with the neuroradiology staff, an effort was made to generate sagittal sections, which corresponded most closely to those of each patient's preoperative MRI (Fig. 2).
SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) for analysis of the paired t-test to compare the relative change in pre- and postoperative neuroforaminal dimension for each patient and each operative level of the lumbar spine.
Twenty-two males and 4 females comprised the study population. All patients underwent surgical intervention as planned without significant complication; 8 patients underwent 1 level fusion (L5-S1); 12 patients had 2 levels (L4-S1) fused; and 6 patients had 3 levels (L3-S1) fused.
Postoperative foraminal cross sectional area was found to be an average of 43.3% larger postoperatively than preoperatively (p < 0.05) when all operative levels were considered together. Increases by level were found to be 19.2% for L3-4, 57.1% for L4-5, and 40.1% for L5-S1 (Table 1).
Average preoperative and postoperative supradjacent foraminal cross sectional areas were statistically unchanged at 125.84 mm2 and 124.89 mm2, respectively. There was no significant difference (p < 0.5) (Fig. 3).
Radiculopathy caused by degenerative disc disease is the one of the most common indications for spine surgery. Treatment options include direct methods of nerve root decompression (e.g., foraminotomy), as well as indirect methods of decompression (e.g., distraction across the segment). Such indirect methods are commonly employed within the cervical spine.1-3) Shen et al.3) compared the clinical outcomes and graft fusions rates in 109 anterior cervical discectomy and fusion (ACDF) patients with or without direct uncovertebral joint decompression. They found comparable rates of good to excellent results in both groups (84.5% with direct decompression, 84.2% without), and concluded that routine direct uncovertebral joint decompression was unnecessary during ACDF.
In contrast, radiculopathy arising from lumbar foraminal stenosis is generally addressed through more direct posteriorly-based techniques.4) Possible reasons for this difference may include the relative safety of the posterior lumbar approach, as well as uncertainty as to the degree of decompression attainable through indirect methods of distraction.
Relatively few authors have sought to determine how much indirect decompression of the lumbar nerve root can be achieved through anterior distraction alone. If the neural foramen could be reliably expanded through indirect means, it is possible that the indications for certain current and future anterior lumbar procedures could be expanded.
Several methods of distraction have been studied in cadaveric and animal models. Infusa et al.5) and Humke et al.6) measured the foraminal heights of cadavers after posterior distraction by pedicle screws and found that increased foraminal dimensions resulted. The minimal amount of posterior distraction necessary to reliably improve foraminal dimensions was suggested to be 6 mm, using pedicle screw instrumentation.
Sandhu et al.7) compared interbody heights in 20 sheep that had undergone ALIFs with either a threaded titanium interbody fusion device, autogenous iliac crest dowel graft, or interbody decortication only. The authors demonstrated that despite early subsidence, interbody fusion cages successfully distracted and preserved interbody spaces. However, they didn't measure the foraminal height. Chen et al.8) were the only authors who measured the foraminal height directly after cage insertion. Their results indicated that anterior systems such as the BAK system, which increase disc heights, can significantly increase neuroforaminal volume and area, providing adequate space for the nerve root and improving neuroforaminal stenosis. However, Chen et al.8) measured foraminal height only in the cadaveric spine.
To our knowledge, no prior study has prospectively assessed the relative change in MRI demonstrated neuroforaminal dimensions in a consecutive series of patients after anterior interbody fusion without any posterior decompression. One of the co-authors was a neuroradiologist, who made an effort to cut both pre- and postoperative MRI at the same sagittal plane to maximize the correspondence. We compared preoperative foraminal dimensions and cross sectional area at each operative level with the corresponding postoperative size to determine the change attributable to anterior distraction alone, in patients undergoing surgery for degenerative disease of the lumbar spine. We observed and report that indirect decompression by anterior distraction can increase neuroforaminal dimension even in the absence of formal posterior distraction or foraminotomy as demonstrated on MRI. Furthermore, there was no significant change in the neuroforaminal dimension immediately above the fused segments. This finding may serve to alleviate some concern that anterior distraction of one lumbar segment necessarily implies relative compression of the level above.
When it comes to foramen dimension measurement, both computed tomography (CT) and MRI can be used. CT can show more bony foramenal shape, while MRI can give us more comprehensive foramenal shape. One of the disadvantage of using MRI is there can be more artifacts especially after instrumentation. In our study, however, all instrumentation was made of titanium and, all MRI exams were performed under a neuroradiologist's supervision. The neurologist is a co-author of this paper.
Our investigation has several major limitations. First, the side used for comparison of foraminal dimension was not standardized. At our center, ALIF is generally performed through a left-sided approach, which results in a predominantly left-sided cut in the annulus fibrosus. This technique potentially results in variability between the right- and left-sided foraminal sizes. An intact right-sided annulus could theoretically act as a tether, limiting the distraction of the right neural foramen. If a distraction had occurred, the left-sided neuroforamina would tend to be larger than those on the right side. We did not compare right- and the left-sided dimensions for individual patients, as the sagittal cuts on both sides were rarely of sufficient quality to enable such a comparison. However, because the side measured postoperatively was always matched to the same side measured preoperatively, this limitation likely did not significantly alter our results.
Also, we are limited by the position in which the MR images were taken. All patients were imaged supine both pre- and postoperatively. Certainly, it is possible that upright MRI may have yielded a numerically different result. In fact, it is likely that an upright MRI may have demonstrated an even greater increase in foraminal cross sectional area than our supine study. With a rigid interbody device in place, there is unlikely to be any substantial change in postoperative foraminal dimension with position. However, we would expect the preoperative foraminal dimension to have been even smaller than that measured supine. It is impossible to quantify to what extent our results underestimate the change that would be appreciated on an upright MRI.
Our patient population is itself a limiting factor. Because the principal surgical indication for our patients was degenerative disk disease with relatively mild resultant foraminal stenosis, the true clinical effect of an average 43.3% increase in foraminal dimension cannot be quantified. Patients whose radiculopathic pain was far more significant than their back pain would certainly not be treated with an indirect decompression alone. The question may be asked, "How much indirect decompression is enough?" For patients with predominantly leg pain, 43.3% may not be enough.
Finally, the point can certainly be made that the taller the interbody graft, the greater the distraction and increase in foraminal size that can result. By allowing each individual surgeon to choose the most appropriate graft size at each level based on clinical judgment, we have not controlled for this variable. It has been our experience that overdistraction of the interbody space may actually result in nerve root tension and a worsening of radicular symptoms. By including multiple-experienced surgeons in this investigation who were aware of the consequences of overdistraction, we sought to minimize the resulting variability. Without having controlled for graft size, we cannot eliminate this limitation of our analysis.
In conclusion, we demonstrated that anterior interbody fusion with a corticocancellous allograft can significantly increase neuroforaminal dimension and cross-sectional area, even in the absence of formal posterior distraction or foraminotomy. In addition, anterior interbody fusion with anterior distraction alone appears to have little effect upon supradjacent, neuroforaminal dimensions as demonstrated on MRI. Despite the limitations inherent to our study, it is our hope that this investigation adds to the debate over the index and the supradjacent segment consequences of ALIF.
Figures and Tables
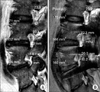 | Fig. 2Measurement of index foraminal dimensions in preoperative (A) and postoperative (B) magnetic resonance imaging. |
References
1. Sekerci Z, Ugur A, Ergun R, Sanli M. Early changes in the cervical foraminal area after anterior interbody fusion with polyetheretherketone (PEEK) cage containing synthetic bone particulate: a prospective study of 20 cases. Neurol Res. 2006. 28(5):568–571.
2. Bartels RH, Donk R, van Azn RD. Height of cervical foramina after anterior discectomy and implantation of a carbon fiber cage. J Neurosurg. 2001. 95:1 Suppl. 40–42.
3. Shen FH, Samartzis D, Khanna N, Goldberg EJ, An HS. Comparison of clinical and radiographic outcome in instrumented anterior cervical discectomy and fusion with or without direct uncovertebral joint decompression. Spine J. 2004. 4(6):629–635.
4. Jenis LG, An HS, Gordin R. Foraminal stenosis of the lumbar spine: a review of 65 surgical cases. Am J Orthop (Belle Mead NJ). 2001. 30(3):205–211.
5. Infusa A, An HS, Glover JM, McGrady L, Lim TH, Riley LH 3rd. The ideal amount of lumbar foraminal distraction for pedicle screw instrumentation. Spine (Phila Pa 1976). 1996. 21(19):2218–2223.
6. Humke T, Grob D, Grauer W, Sandler A, Dvorak J. Foraminal changes with distraction and compression of the L4/5 and L5/S1 segments. Eur Spine J. 1996. 5(3):183–186.
7. Sandhu HS, Turner S, Kabo JM, et al. Distractive properties of a threaded interbody fusion device: an in vivo model. Spine (Phila Pa 1976). 1996. 21(10):1201–1210.
8. Chen D, Fay LA, Lok J, Yuan P, Edwards WT, Yuan HA. Increasing neuroforaminal volume by anterior interbody distraction in degenerative lumbar spine. Spine (Phila Pa 1976). 1995. 20(1):74–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download