Abstract
Background
The purpose of the present study was to compare the clinical results of 3 posterior cruciate ligament reconstruction techniques according to the time from injury to surgery and remnant PCL status and to evaluate the efficiency of each technique.
Methods
The records of 89 patients who underwent primary PCL reconstructions with a posterolateral corner sling were analyzed retrospectively. Thirty-four patients were treated by anterolateral bundle (ALB) reconstruction with preservation of the remnant PCL using a transtibial tunnel technique in the acute and subacute stages of injury (group 1). Forty patients were treated with remnant PCL tensioning and an ALB reconstruction using the modified inlay technique in the chronic stage (group 2), and fifteen patients were treated with double-bundle reconstruction using the modified inlay technique (group 3). The double-bundle reconstruction was performed if there was a very weak or no PCL remnant.
Results
The mean side-to-side differences in posterior tibial translation on the stress radiographs were reduced from 10.1 ± 2.5 mm in group 1, 10.6 ± 2.4 mm in group 2, and 12.8 ± 3.2 mm in group 3 preoperatively to 2.3 ± 1.4 mm in group 1, 2.3 ± 1.5 mm in group 2, and 4.0 ± 2.5 mm in group 3 at the last follow-up (p < 0.001, p < 0.001, and p < 0.001, respectively). Statistical analyses revealed that group 1 and group 2 were similar in terms of side-to-side difference changes in posterior tibial translation on the stress radiographs; however, group 3 was inferior to group 1 and group 2 at the last follow-up (p = 0.022). The clinical results were not significantly different among the three groups.
Conclusions
Excellent posterior stability and good clinical results were achieved with ALB reconstruction preserving the injured remnant PCL in the acute and subacute stages and remnant PCL tensioning with ALB reconstruction in the chronic stage. The PCL injuries could be surgically corrected with different techniques depending on both the remnant PCL status and the interval between the knee trauma and operation.
In recent years, the incidence of posterior cruciate ligament (PCL) injuries has increased, possibly due to more frequent participation in recreational and competitive sports. Consequently, arthroscopic PCL reconstructions have increased, which has been facilitated by the awareness of the pathogenesis, clinical diagnostic skills, and development of arthroscopic instruments and surgical techniques that have enhanced clinical outcomes.1-3) However, the most effective arthroscopic PCL reconstruction method has not been definitively determined, and some controversy remains as to whether to employ a single or double femoral tunnel, a 1- or 2-incision technique, and a transtibial tunnel or tibial inlay technique.3-5) Furthermore, consensus on the ideal PCL reconstruction timing for good postoperative stability and functional scores does not exist, despite several authors recommending an early rather than late PCL reconstruction.6,7)
PCL injuries have the potential for intrinsic healing, and several magnetic resonance imaging (MRI) studies reported that PCL healed with continuity but with residual laxity in their cases.8-10) The remaining PCL structures have the potential benefit of enhanced revascularization, preservation of proprioceptive function through the mechanoreceptors in the original PCL, as well as addition to the mechanical stability of the knee joint.11-14) In addition, the identification of concomitant injuries of the posterolateral corner (PLC) is important for optimizing the surgical and clinical outcomes.15-18)
In the present study, we compared the clinical results of the following three techniques after two years of follow-up: 1) a remnant PCL-preserving stent (anterolateral bundle [ALB] reconstruction) procedure with the transtibial tunnel technique in the acute or subacute stage; 2) tensioning of the remnant PCL using the modified tibial inlay technique with a single-bundle reconstruction in the chronic stage; and 3) a PCL double-bundle reconstruction with a modified tibial inlay technique. We hypothesized that PCL reconstruction surgery in the chronic stage would yield inferior results in terms of stability and clinical scores compared to PCL reconstruction in the acute or subacute stage. Additionally, PCL reconstruction procedures with remnant preservation would have benefits in terms of the stability and functional outcomes.
A retrospective, comparative cohort study was conducted on patients who underwent 152 primary PCL reconstruction surgeries performed by a single surgeon between April 2004 and October 2008. Grade II PCL injuries combined with other ligamentous or grade III PCL injuries were considered for surgical treatment when the patients had persistent pain or functional disability (discomfort when going down stairs). In this situation, the status of the remnant PCL tissue (determined using MRI) was considered before surgery in order to select the most appropriate reconstruction option. The remnant PCL-preserving stent procedure using the transtibial tunnel technique was chosen if there was partial continuity or the remnant tissue was sufficient in the acute (< 3 months) or subacute stage (3-6 months). Remnant PCL tensioning and ALB reconstruction with the modified inlay technique was selected if there was abundant remnant tissue with continuity for tensioning in the chronic stage (> 6 months). A double-bundle reconstruction was performed if there was no remnant PCL or a very weak PCL remnant according to the MRI and arthroscopic findings in the subacute or chronic stage.
Thirty-three patients were excluded because they met one of the following exclusion criteria: 1) concomitant anterior cruciate ligament (ACL) or medial collateral ligament reconstruction (12 patients); 2) an associated fracture in the lower extremities that could affect knee function (2 patients); 3) combined severe life-threatening medical disease (1 patient); and 4) revision surgery (8 patients). Moreover, ten patients were also excluded because of loss to outpatient follow-up before 24 months.
The grading of PLC injuries has been described elsewhere (Table 1).15,19) To minimize the effect of combined PLC injuries, only patients with grade II PLC injury treated by PLC sling (modified Larson technique) simultaneously with a PCL reconstruction were enrolled in the present study. Twenty patients, assessed as having grade I or III PLC injuries, were excluded (Table 2). Therefore, the records of 89 patients, who had been followed up for a minimum of 24 months after PCL reconstruction were included in the analysis.
The study was approved by the Institutional Review Board at Chung-Ang University Hospital, and all patients provided informed consent. Out of 89 patients, 34 were treated with a PCL remnant-preserving ALB reconstruction using the transtibial tunnel technique in the acute and subacute stage of injury (group 1); 40 patients were treated with remnant tensioning and ALB reconstruction using the modified inlay technique in the chronic stage of injury (group 2); and 15 patients were treated with double-bundle reconstruction using the modified inlay technique (group 3). The mean age of patients in group 1 (32 males and 2 females), group 2 (36 males and 4 females), and group 3 (14 males and 1 female) was 31.1 years (range, 18 to 51 years), 31.2 years (range, 16 to 59 years), and 32.1 years (range, 19 to 24 years), respectively. An Achilles tendon allograft was used in the PCL double-bundle reconstruction and an autogenous hamstring tendon was used in the other two groups. The PLC reconstruction was performed with a contralateral autogenous hamstring tendon through the fibular head tunnel in all patients.
The surgical technique has been described elsewhere6,20) and we describe only the specific technical aspects of arthroscopically-assisted PCL reconstruction here. In order to prepare the femoral tunnel without removing the remains of the PCL, the surgeon placed the tip of the guide pin on the PCL at the 12:30 to 1 o'clock position for the right knee while looking through the anterolateral portal. After placing the femoral guide (Acufex Microsurgical, Mansfield, MA, USA) 5 to 6 mm proximal to the margin of the articular cartilage of the medial femoral condyle at the pin, a tunnel was made using the outside-in technique. A posteromedial portal was made to ensure proper preparation of the tibial tunnel. A switching stick was then inserted through the posteromedial portal via an arthroscope sleeve to reach the septum. Under direct visualization, the stick was gently pushed into the posterolateral compartment by piercing the posterior septum just posterior to the PCL remnant and then it was pushed until it reached the capsule at the posterolateral portal site. A skin incision was made at this point, which was approximately 1 cm posterior to the lateral femoral condyle and 1 cm proximal to the joint line, to create the posterolateral portal (modified transeptal portal). A motorized shaver was inserted through the posteromedial portal to reach the posteromedial compartment under direct visualization of the arthroscope which had been inserted earlier through the posterolateral portal. The posterior capsule was carefully separated from the PCL with the shaver and electric VAPR (DePuy Mitek, Raynham, MA, USA), starting from the articular surface level to the tibial attachment, without disrupting the remnant PCL bundle. It was important to keep the shaver's cutting surface in the anteroinferior direction to avoid damaging any posterior neurovascular structure. While looking arthroscopically through the posteromedial or posterolateral portal using a C-arm, the surgeon introduced the tip of the PCL tibial guide (Acufex Microsurgical) through the anteromedial portal and advanced it to the back side of the PCL. The surgeon positioned the guide 1.5 cm below the articular surface just distal to the tibial insertion of the PCL in order to avoid damaging the remnant PCL attachment. The drill guide angle of the tibia was oriented at 55° to 60°, and the tunnel diameter was determined as the same size as the graft diameter. The autogenous hamstring quadruple graft was passed through the femoral tunnel first, and then passed through the tibial tunnel with secured about the soft tissue impingement. For the tibial side fixation, a Rigidfix guide (DePuy Mitek) insert and two cross pin guides were placed into the lateral side of the tibial plateau. The graft tendon was fixed at the tibial tunnel, first with Rigidfix. After the tension and accurate positioning of the graft were assessed arthroscopically, the graft was cyclic loaded in tension with 15 to 20 lb and fixed with a biodegradable interference screw at the femoral tunnel additionally, and a post and tie were made with a screw and washer in both the tibial and femoral sides with the knee joint flexed at 90°.
The surgical technique has been described elsewhere,19,21-24) and we only describe the specific technical aspects of the arthroscopically-assisted PCL reconstruction here. The femoral tunnel was made in the same manner as the transtibial tunnel (stent operation). For the posterior approach, the knee is flexed 70° to 90° to provide easier access to the popliteal area and the operating table is tilted over 30° so that the operated knee is lower than the contralateral knee. After the posteromedial approach,25) the PCL tibial attachment was demarcated as a 1.5 × 2 cm area using an osteotome, and a 7-mm-thick bone block was detached from the distal to the proximal area using a 1.2 to 1.5 cm wide curved osteotome. A bony trough was made at the medial side of the PCL tibial insertion just distal to the portion of the detached bone block site to which the detached bone block and graft were to be fixed. The autogenous hamstring quadruple graft was passed through the knee joint to the femoral tunnel and fixed to the cortical bone of the distal and medial side of the tibial insertion of the PCL with a 10-mm staple. The remnant PCL was tensioned by pulling distally and the step-off as an anatomic reduction was confirmed at the anteromedial aspect of the tibiofemoral condyle. The remnant PCL was fixed through the tibia using a 5- or 6.5-mm cannulated screw with a spiked washer. The graft was cyclically loaded in tension with 15 to 20 lb of tension and fixed with a biodegradable interference screw at the femoral tunnel with the knee joint flexed 70° to 90° and additionally fixed with a 10-mm staple or post-tie on the femoral side.
For PCL double-bundle reconstruction surgery, the double femoral tunnel and modified tibial inlay technique with an allograft tendon was employed in all 15 patients. For the allograft, a tibial bone block with two femoral bundles was prepared. The Achilles tendon graft was transected sagittally into two strands, each sutured with No. 5 non-absorbable sutures using a locking whipstitch to to be 6 to 8 mm in diameter (Fig. 1). The ALB was made larger than the posteromedial bundle (PMB). The Achilles tibial bone block was 20 mm long, 15 mm wide, and 7 to 8 mm thick.
The femoral tunnel of the ALB was made in the same manner as the other two techniques. The femoral tunnel of the PMB was made from an accessory anterolateral portal using an inside-out technique, where the desired femoral tunnel was placed 8 mm proximal to the margin of the articular cartilage of the medial femoral condyle at the 3 (right knee) or 9 o'clock position (left knee) (Fig. 2). The original PCL remnant bundle was preserved where possible. Tibial fixation was achieved in the same manner with a modified inlay operation. After the posteromedial approach with a tilting operation table and lower down affected side which was flexion, abduction and external rotation of the hip and 90° flexion of the knee position on the ancillary table, the tibial attachment point of the PCL was demarcated and a bony trough was made just distal to the PCL tibial insertion where the detached bone block was to be fixed. The two femoral Achilles allograft bundles were passed through the knee joint to the femoral tunnel; the PMB was passed first, followed by the ALB. The Achilles bone block was fixed to the tibia using a 5- or 6.5-mm cannulated screw with a spiked washer.
The postoperative rehabilitation regimen has been described elsewhere,6,19) and it was customized for each patient depending on the rigidity of graft fixation, degree of isometricity, and results of the final intraoperative stress test after the reconstruction. Since the PLC reconstructions were performed at the same time as the PCL reconstructions, passive range of motion exercises usually started on the 3rd to 5th postoperative day. During the first six weeks, the range of motion was increased from 0° to 90° and the full range of motion was regained by the 12th to 24th postoperative week. The knee was immobilized with a long leg removable splint for two weeks after surgery. The PCL brace,26) which is made up with a tibial supporter including an elastic spring at the posterior aspect of the proximal tibia, was used until the 6th postoperative week. Full weight bearing without the use of crutches was generally achieved within the 8th postoperative week.
To compare the clinical and stability outcomes of the PCL reconstructions, serial postoperative evaluations were performed at 6 weeks, 3, 6, and 12 months, and every 12 months thereafter. Knee stability was evaluated by posterior stress radiography (push view) performed using a Telos stress device (Austin & Associates, Fallston, MD, USA) and by a maximum manual displacement test using a KT-1000 arthrometer (Instrumented Drawer Testing, KT-1000, MEDmetric, San Diego, CA, USA). The tests were performed preoperatively and at every follow-up after the 3rd postoperative month. The subjective and objective International Knee Documentation Committee (IKDC) score and Orthopädische Arbeitsgruppe Knie (OAK) score were used for the clinical evaluation. All evaluations were made by a single observer not involved in the operation. To test posetrolateral rotator instability, the posterolateral drawer test, dial test, and varus stress test were performed at every follow-up, and the results were compared with the results from the contralateral side, and classified as overconstrained, constrained and lax.19)
A power analysis was performed. If the subjective scores differed by more than 10 points and the posterior stress radiograph results differed by more than 2 mm, they were considered clinically different results. To achieve a power of 80% under a significance level of 5%, a sample size of 14 cases was needed for each study group. The Wilcoxon signed rank was used to analyze the pre- and postsurgery data. The statistical comparison between the 3 groups was made using a Kruskal-Wallis test. All statistical analyses were performed using SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA), and SAS ver. 9.1 (SAS Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
For the 34 patients in group 1, the mean side-to-side difference in posterior translation, as measured by posterior stress radiography, improved from 10.1 ± 2.5 mm preoperatively to 2.3 ± 1.4 mm at the last follow-up (p < 0.001). At the last evaluation, 23 patients (67.1%) exhibited a displacement of less than 3 mm, 8 patients (23.5%) had between a 3 and 5 mm displacement, and 3 patients (8.8%) showed displacement exceeding 5 mm (Fig. 3). The mean side-to-side difference as measured by the maximal manual test using the KT-1000 arthrometer also improved from 6.8 ± 2.0 mm preoperatively to 2.2 ± 2.2 mm at the last follow-up (p < 0.001).
For 40 patients in group 2, the mean side-to-side difference in posterior translation, as measured by posterior stress radiography, improved from 10.6 ± 2.4 mm preoperatively to 2.3 ± 1.5 mm at the last follow-up (p < 0.001). At the last evaluation, 23 patients (57.5%) exhibited a displacement of less than 3 mm, 15 patients (37.5%) had between a 3 and 5 mm displacement, and 2 patients (5.0%) showed displacement exceeding 5 mm. The mean side-to-side difference, as measured by the maximal manual test using the KT-1000 arthrometer, also improved from 8.4 ± 2.2 mm preoperatively to 2.0 ± 1.4 mm at the last follow-up evaluation (p < 0.001).
For the 15 patients in group 3, the mean side-to-side difference in posterior translation, as measured by posterior stress radiography, improved from 12.8 ± 3.2 mm preoperatively to 4.0 ± 2.5 mm at the last follow-up (p < 0.001). At the last evaluation, 3 patients (20%) exhibited a displacement of less than 3 mm, 9 patients (60%) had between a 3 and 5 mm displacement, and 3 patients (20%) showed displacement exceeding 5 mm. The mean side-to-side difference, as measured by the maximal manual test using the KT-1000 arthrometer, also improved, from 7.7 ± 2.2 mm preoperatively to 3.6 ± 1.9 mm at the last follow-up (p < 0.001).
One case in group 1 and one in group 3 showed a displacement greater than 10 mm compared to the normal side. Statistical analyses revealed both group 1 and 2 showed similar posterior stress radiography results or KT-1000 arthrometer stability. However, the results of group 3 were inferior to groups 1 and 2 at the last follow-up evaluation (p = 0.022).
At the last evaluation, rotational stability was assessed according to different knee flexion values (30° and 90° of flexion): 4 knees were overconstrained, 27 were constrained, and 3 were lax as compared with the normal side in group 1. In group 2, 6 knees were overconstrained, 31 were constrained, and 3 were lax. In group 3, 3 knees were overconstrained, 9 were constrained and 3 were lax. There were no statistical differences among the three groups in terms of rotational stability (p = 0.214).
The mean OAK score improved significantly for the 34 patients in group 1 from 71.7 ± 9.3 preoperatively to 85.0 ± 6.7 postoperatively (p < 0.001). The mean IKDC subjective score improved significantly from 46.7 ± 16.6 preoperatively to 65.1 ± 18.4 postoperatively (p < 0.001). With regard to the IKDC objective evaluation, 11 patients (32.4%) and 23 patients (67.6%) were rated as C (abnormal) and D (severely abnormal), respectively, at the preoperative evaluation. At the last evaluation, 21 patients (61.8%), 9 patients (26.5%), 3 patients (8.8%), and 1 patient (2.9%) were rated as A (normal), B (nearly normal), C and D, respectively (Fig. 4). Therefore, 88.3% of the patients had a rating of either A or B at the last evaluation.
For the 40 patients in group 2, the mean OAK score improved significantly from 63.5 ± 10.4 preoperatively to 88.9 ± 7.6 postoperatively (p < 0.001). The mean IKDC subjective score improved significantly from 63.5 ± 10.4 preoperatively to 79.7 ± 13.3 postoperatively (p < 0.001). With regard to the final IKDC evaluation, 13 patients (32.5%) and 27 patients (67.5%) were rated as C and D, respectively, at the preoperative evaluation. At the last evaluation, 17 patients (42.5%), 19 patients (47.5%), and 4 patients (10%) were rated as A, B, and C, respectively. Hence, 90% of the patients had a rating of either A or B at the last evaluation.
The mean OAK score for the 15 patients in group 3 improved significantly from 71.3 ± 12.9 preoperatively to 83.0 ± 5.9 postoperatively (p < 0.001). The mean IKDC subjective score improved significantly from 50.7 ± 17.6 preoperatively to 61.5 ± 12.9 postoperatively (p < 0.001). With regard to the final IKDC evaluation, 6 patients (40%) and 9 patients (60%) were rated as C and D, respectively, at the preoperative evaluation. At the last evaluation, 3 patients (20%), 9 patients (60%), 2 patients (13.3%), and 1 patient (6.7%) were rated as A, B, C, and D, respectively. Hence, 80% of the patients had a rating of either A or B at the last evaluation (Fig. 4). There was no a significant difference among the three groups in terms of the clinical results, namely the OAK score and IKDC objective and subjective scores.
The management of PCL injuries remains a challenging clinical problem. The present study evaluated the outcomes of three PCL reconstruction procedures applied according to our PCL treatment algorithm. The most important finding of this study was that combined PCL-PLC instabilities can be treated successfully using different PCL reconstruction techniques, depending on the PCL remnant status, combined with a reconstruction of the PLC structures. Although the three groups did not differ in terms of clinical outcomes, the posterior knee stability of the double-bundle reconstruction group (group 3) was inferior to the single bundle reconstruction groups (groups 1 and 2).
In 2004, our institute began using the same PCL treatment algorithm.21) If the PCL injury at the acute or subacute stage was grade II or less in severity and other ligament injuries (e.g., medial collateral injury or PLC injuries) were absent, active conservative treatment was initiated using anti-sagging cast immobilization and a PCL brace.26) Subsequently, the stability and function of the injured knee were reevaluated at the end of the conservative treatment period. Conversely, for grade II injuries combined with other ligament injuries or for grade III injuries, surgical treatment was considered even in the subacute stage, according to the activity level and patient demand.
In the present study, we could not compare the results according to the timing and different techniques were applied according to the stage (acute or chronic). However, the remnant PCL fibers were tensioned and the ALB was augmented if there was abundant remnant PCL for tensioning in the chronic cases at least six months after the initial PCL injury in this series. It was hypothesized that the remnant PCL fibers could play an important role in posterior knee stability and clinical outcomes.
Several studies on cadavers have shown that double-bundle PCL reconstruction is biomechanically superior to single-bundle PCL reconstruction.27,28) Furthermore, Kim et al.3) reported that arthroscopic tibial inlay double-bundle PCL reconstruction resulted in better posterior stability than did the two single-bundle methods. However, this present study showed that the posterior knee stability of the double bundle reconstruction group was inferior to those of the single bundle reconstruction group although the three groups did not differ in terms of the clinical outcomes. This may be because of several reasons. First, the initial status of the patients in the double bundle group might have had more severe instability than those in the other two groups. A double bundle reconstruction was only performed if there was no remnant PCL or a very weak PCL remnant in the subacute or chronic stage. In addition, most of the patients in the double bundle group had combined associated injuries although only patients with grade II PLC injury were enrolled in this series. The second possible reason is that the Achilles allograft, which may have disadvantages including the possible tendency of elongation and delayed revascularization, was only used in the double bundle group although hamstring autografts were prepared in the other two groups. Therefore, the augmentation procedure for the ALB using the double stranded autogenous semitendinosus may be needed if the Achilles allograft is not strong enough for the double bundle reconstruction after preparation.
The PCL had better synovial coverage, blood circulation, and potential to spontaneously heal than the ACL. Numerous MRI studies8-10) and an experimental animal study11) reported that an acute ruptured PCL has the potential to heal. Therefore, the PCL appears to have spontaneous healing potential even in the cases of rupture of the substance, but laxity often remains. The results of the remnant preservation technique as both a stent procedure in the acute or subacute stage and tensioning of the remnant PCL in the chronic stage, particularly in terms of stability, were improved considerably compared to the double-bundle PCL reconstruction procedure. Safran et al.13) reported that the mechanoreceptors in PCL-injured knees act as knee stabilizers, which explains why patients with posterior instability develop degenerative changes more slowly than patients with anterior instability. We theorized that if the remnant PCL is not removed but preserved, or tensioning is performed surgically, there could be an advantage potentially in enhancing synovialization and in preserving the proprioceptive function of the mechanoreceptor in a continuous PCL as well as in conferring stability similar to a normal PCL.11,13) Therefore, we would recommend that the PCL remnant be preserved as much as possible, including avoiding sacrifice of the PCL remnant during PCL surgery. Furthermore, PCL reconstruction could be applied with different techniques depending on the PCL remnant status and post trauma stage. However, long-term studies and a new functional system which can reflect proprioception will be needed to demonstrate the potential benefit of these surgical methods.
Several limitations exist in the present study. First, the study was a non-randomized retrospective study, and a proprioceptive function test was not performed. Therefore, the results do not indicate whether the PCL remnant preservation technique enhances the clinical outcome with regard to proprioception. Prospective studies, including assessment of the correlations with the clinical outcomes, and possible long-term follow-up will be needed. Second, the overall number of cases was small and the sample sizes were not uniform across the three groups. Furthermore, the initial status of the patients in the three groups was not equivalent because the PCL surgery was applied according to different surgical indications. Third, the selection of the autograft or allograft, and the fixation methods were different for each group, potentially affecting the healing potential and stability. Nevertheless, an advantage of the present study was that all PCL operations were performed by a single surgeon using a standardized surgical technique, in order to minimize the treatment variables. Moreover, multiple knee scores as well as stress radiographs were used to evaluate the method. Finally, combined PLC injuries may have influenced the results among three groups. However, only patients with a grade II PLC injury were enrolled in the present study, to minimize the effect of combined PLC injuries.
Excellent posterior stability and good clinical results were achieved with an ALB reconstruction, with preservation of the injured remnant PCL in the acute and subacute stages, and remnant PCL tensioning with ALB reconstruction in the chronic stage. PCL injuries could be surgically corrected with different techniques, depending on both the remnant PCL status and the interval between the knee trauma and operation.
Figures and Tables
Fig. 1
An Achilles tendon allograft is prepared. A tibial bone block and two tendon tails are secured with No. 5 non-absorbable sutures using a locking whipstitch.

Fig. 2
A guide pin is located 5 to 6 mm proximal to the margin of the articular cartilage of the medial femoral condyle (MFC) for the anterolateral bundle (black arrow) and 8 mm proximal to the margin of the articular cartilage for the posteromedial bundle (white arrow). ACL: anterior cruciate ligament.
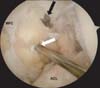
Fig. 3
Evaluation of posterior tibial translation on posterior stress radiographs at the last follow-up in the three groups. The results of the posterior cruciate ligament (PCL) double-bundle group were inferior to the PCL stent and tensioning groups.

References
1. Ahn JH, Yang HS, Jeong WK, Koh KH. Arthroscopic transtibial posterior cruciate ligament reconstruction with preservation of posterior cruciate ligament fibers: clinical results of minimum 2-year follow-up. Am J Sports Med. 2006; 34(2):194–204.

2. Heinzelmann AD, Barrett GR. Posterior cruciate ligament reconstruction: Achilles tendon allograft, double bundle. Clin Sports Med. 2009; 28(2):245–257.

3. Kim SJ, Kim TE, Jo SB, Kung YP. Comparison of the clinical results of three posterior cruciate ligament reconstruction techniques. J Bone Joint Surg Am. 2009; 91(11):2543–2549.
4. Houe T, Jorgensen U. Arthroscopic posterior cruciate ligament reconstruction: one- vs. two-tunnel technique. Scand J Med Sci Sports. 2004; 14(2):107–111.
5. Shon OJ, Lee DC, Park CH, Kim WH, Jung KA. A comparison of arthroscopically assisted single and double bundle tibial inlay reconstruction for isolated posterior cruciate ligament injury. Clin Orthop Surg. 2010; 2(2):76–84.
6. Jung YB, Jung HJ, Song KS, Kim JY, Lee HJ, Lee JS. Remnant posterior cruciate ligament-augmenting stent procedure for injuries in the acute or subacute stage. Arthroscopy. 2010; 26(2):223–229.
7. Kurtz CA, Sekiya JK. Treatment of acute and chronic anterior cruciate ligament-posterior cruciate ligament-lateral side knee injuries. J Knee Surg. 2005; 18(3):228–239.
8. Jung YB, Jung HJ, Yang JJ, et al. Characterization of spontaneous healing of chronic posterior cruciate ligament injury: analysis of instability and magnetic resonance imaging. J Magn Reson Imaging. 2008; 27(6):1336–1340.
9. Mariani PP, Margheritini F, Camillieri G, Bellelli A. Serial magnetic resonance imaging evaluation of the patellar tendon after posterior cruciate ligament reconstruction. Arthroscopy. 2002; 18(1):38–45.
10. Shelbourne KD, Jennings RW, Vahey TN. Magnetic resonance imaging of posterior cruciate ligament injuries: assessment of healing. Am J Knee Surg. 1999; 12(4):209–213.
11. Jung YB, Lee TJ, Yang DL, Kim KS, Ko KW, Chung JW. Healing potential of the transected posterior cruciate ligament of the rabbit. J Korean Orthop Assoc. 2001; 36(1):25–32.
12. Bray RC, Leonard CA, Salo PT. Vascular physiology and long-term healing of partial ligament tears. J Orthop Res. 2002; 20(5):984–989.
13. Safran MR, Allen AA, Lephart SM, Borsa PA, Fu FH, Harner CD. Proprioception in the posterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 1999; 7(5):310–317.
14. Scapinelli R. Comment on W. Petersen and B. Tillmann, "Blood and lymph supply of the posterior cruciate ligament". Knee Surg Sports Traumatol Arthrosc. 1999; 7(5):337.
15. Jung YB, Jung HJ, Kim SJ, et al. Posterolateral corner reconstruction for posterolateral rotatory instability combined with posterior cruciate ligament injuries: comparison between fibular tunnel and tibial tunnel techniques. Knee Surg Sports Traumatol Arthrosc. 2008; 16(3):239–248.
16. Sekiya JK, Kurtz CA. Posterolateral corner reconstruction of the knee: surgical technique utilizing a bifid Achilles tendon allograft and a double femoral tunnel. Arthroscopy. 2005; 21(11):1400.
17. Wang CJ, Chen HS, Huang TW, Yuan LJ. Outcome of surgical reconstruction for posterior cruciate and posterolateral instabilities of the knee. Injury. 2002; 33(9):815–821.
18. Jung YB, Nam CH, Jung HJ, Lee YS, Ko YB. The influence of tibial positioning on the diagnostic accuracy of combined posterior cruciate ligament and posterolateral rotatory instability of the knee. Clin Orthop Surg. 2009; 1(2):68–73.
19. Lee KH, Jung YB, Jung HJ, et al. Combined posterolateral corner reconstruction with remnant tensioning and augmentation in chronic posterior cruciate ligament injuries: minimum 2-year follow-up. Arthroscopy. 2011; 27(4):507–515.
20. Wang CJ, Chan YS, Weng LH. Posterior cruciate ligament reconstruction using hamstring tendon graft with remnant augmentation. Arthroscopy. 2005; 21(11):1401.
21. Jung YB, Jung HJ, Tae SK, Lee YS, Yang DL. Tensioning of remnant posterior cruciate ligament and reconstruction of anterolateral bundle in chronic posterior cruciate ligament injury. Arthroscopy. 2006; 22(3):329–338.
22. Jung YB, Jung HJ, Lee YS, Lee SH, Park YU. Management of concomitant posterolateral rotatory instability and anterior cruciate ligament injuries of the knee. J Korean Orthop Assoc. 2005; 40(5):560–565.
23. Lee SH, Jung YB, Lee HJ, Jung HJ, Kim SH. Revision posterior cruciate ligament reconstruction using a modified tibial-inlay double-bundle technique. J Bone Joint Surg Am. 2012; 94(6):516–522.
24. Jung YB, Jung HJ, Tae SK, Lee YS, Lee KH. Reconstruction of the posterior cruciate ligament with a mid-third patellar tendon graft with use of a modified tibial inlay method. J Bone Joint Surg Am. 2005; 87:Suppl 1. (Pt 2):247–263.
25. Berg EE. Posterior cruciate ligament tibial inlay reconstruction. Arthroscopy. 1995; 11(1):69–76.
26. Jung YB, Tae SK, Lee YS, Jung HJ, Nam CH, Park SJ. Active non-operative treatment of acute isolated posterior cruciate ligament injury with cylinder cast immobilization. Knee Surg Sports Traumatol Arthrosc. 2008; 16(8):729–733.
27. Harner CD, Janaushek MA, Kanamori A, Yagi M, Vogrin TM, Woo SL. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000; 28(2):144–151.
28. Race A, Amis AA. PCL reconstruction: in vitro biomechanical comparison of 'isometric' versus single and double-bundled 'anatomic' grafts. J Bone Joint Surg Br. 1998; 80(1):173–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download