Abstract
Background
In primary total hip replacements (THRs), the dissected femoral heads (FHs) are commonly used to make the bone-chips for the reconstruction in the orthopaedic surgery. The donated FHs are routinely microbiologically cultured to identify and contaminated FHs are discarded. This study examines whether a positive FH culture predicts an infection and prosthetic failure after primary THR.
Methods
The study sampled 274 donated FHs from patients with osteonecrosis (ON), hip joint osteoarthritis (OA), and femoral neck fracture (FNF) in THR to culture the microbes. The FH contamination rates were analyzed for ON, OA, and FNF groups. Proportion of the postoperative infection or prosthetic failure in the group of donors with a positive FH culture were compared to the proportion in the group of donors with a negative FH culture.
Results
The rates of the positive culture in the ON, OA, and FNF groups were 7.1%, 3.8%, and 4.0%, respectively. The infection rate was found to be non-significantly greater in the ON group than in the OA and FNF groups. In the negative culture group, one patient (0.63%) had a postoperative superficial infection, and five patients (3.2%) experienced additional surgeries including a fixation for a periprosthetic fracture, within a minimum follow-up of two years. However, no postoperative infection was encountered, and no revision surgery was required in the positive culture group.
Conclusions
A positive FH culture is not always associated with elevated risks of infection or prosthetic failure after THR. Therefore, such finding cannot be used as a prognostic factor of THR. The FHs that return a positive culture may not lead to the orthopaedic assessment of an infection or other postoperative complication risks in primary THR.
The femoral heads (FHs) obtained during primary total hip replacement (THR) surgery are commonly used for reconstruction in orthopaedic surgery. To minimize the potential risks of the infection transmission via implantation of the bone allograft, the donated FHs are collected and screened in accordance with the stringent bone banking guidelines. In particular, the sterility of the donated FHs is routinely tested by the microbacterial culture in the bone banks, and the FHs that return positive cultures are discarded.
The reported rates of bacterial contamination among the FHs procured from living donors range from 1% to 22% according to the studies by Chapman and Villar,1) Ivory and Thomas,2) and Tomford et al.3) The differences in the reported contamination rates may result from the number of specimen cultured, sampling techniques and the bacterial screening methods used in the different bone banks. Therefore, the interpretation of the FH microbacterial culture findings is difficult, especially with regards to the false positives and false negatives, and the clinical significance of the positive culture for the donor with respect to the potential infection of the newly implanted prosthetic joint.4-7) The positive FH culture could represent contamination of the sample intraoperatively or during the sampling or tissue culture process. It could also indicate a pre-existing infection, such as osteomyelitis. In particular, in patients with FH osteonecrosis, the osteonecrotic environment may facilitate the localization and proliferation of bacteria, which raises the questions as to whether FH osteonecrosis increases the likelihood of culture positivity. Furthermore, determination of the source of an infection as contamination or osteomyelitis could provide the means of predicting the risks of wound or hip joint infection after THR. This study was conducted to determine whether the FH positive culture predicted infection and prosthetic failure after primary THR.
There were 273 patients (210 males and 63 females) with osteonecrosis (ON), osteoarthritis (OA), or femoral neck fracture (FNF) who underwent primary THR. The mean patient age was 68.8 years (range, 20 to 88 years). The patients who met the selection criteria (Table 1) were asked to consent to the donation of their dissected FHs. Patients with a recent history of antibiotic use or open trauma were excluded. From January 2005 to February 2010, 1,822 THRs were performed. Of the 1,822 THRs, 274 FHs (15%) were selected for the study. Of the total 1,822 THRs, 1,548 FHs (85%) were excluded as they did not meet the selection criteria.
Two hundred and seventy-four FHs from 273 patients were procured: 169 ON, 80 OA, and 25 FNF. The FHs were sampled at three sites under the strict sterile conditions in the operating theatre immediately after removal: a small piece of the cancellous bone and two FH inner and surface swabs. Specimens were immediately sent to the microbiology laboratory for processing. In the laboratory, under the laminar airflow, the swabs were streaked onto blood agar and inoculated into the liquid thioglycolate broth. The FH fragment was inoculated in the Wilkins-Chalgren broth. All media were incubated at 36℃ under 5% CO2 for 7 days and monitored daily. The blood agar plates were observed closely for growth, and the isolates were identified at the species level. If a broth became turbid during incubation, a subculture was performed on blood agar and the bacteria isolated. The microorganisms retrieved in the cultures were identified at the species level based on the growth condition, colony morphology, Gram staining, acid fast bacteria staining and the urease, catalase and oxidase tests.
Symptoms of infection in donors after THR, such as fever, superficial infection (wound discharge with edema or erythema, and prolonged wound healing), deep infection (purulent discharge, and wound breakdown) or prosthetic infection requiring a revision surgery within two years were evaluated and followed up. All operations and sampling were carried out in a well-equipped operating theatre under strict aseptic conditions. At the induction of anaesthesia, cefazoline 2 g was intravenously administered for prophylaxis. Postoperatively, intravenous antibiotics were routinely administered for 48 hours. Positive microbiological culture rates in the three groups of FHs procured (ON, OA, and FNF groups) were determined, and the relations were sought between the contaminated FHs and the postoperative signs of infection or prosthetic failure. We used the statistical program Microsoft Office Excel 2007. With a sample size > 20, the chi-square statistics was used to test the association between the disease groups with ON, OA, and FNF and the risk factor (contamination of FH). Statistical significance was accepted for p < 0.05.
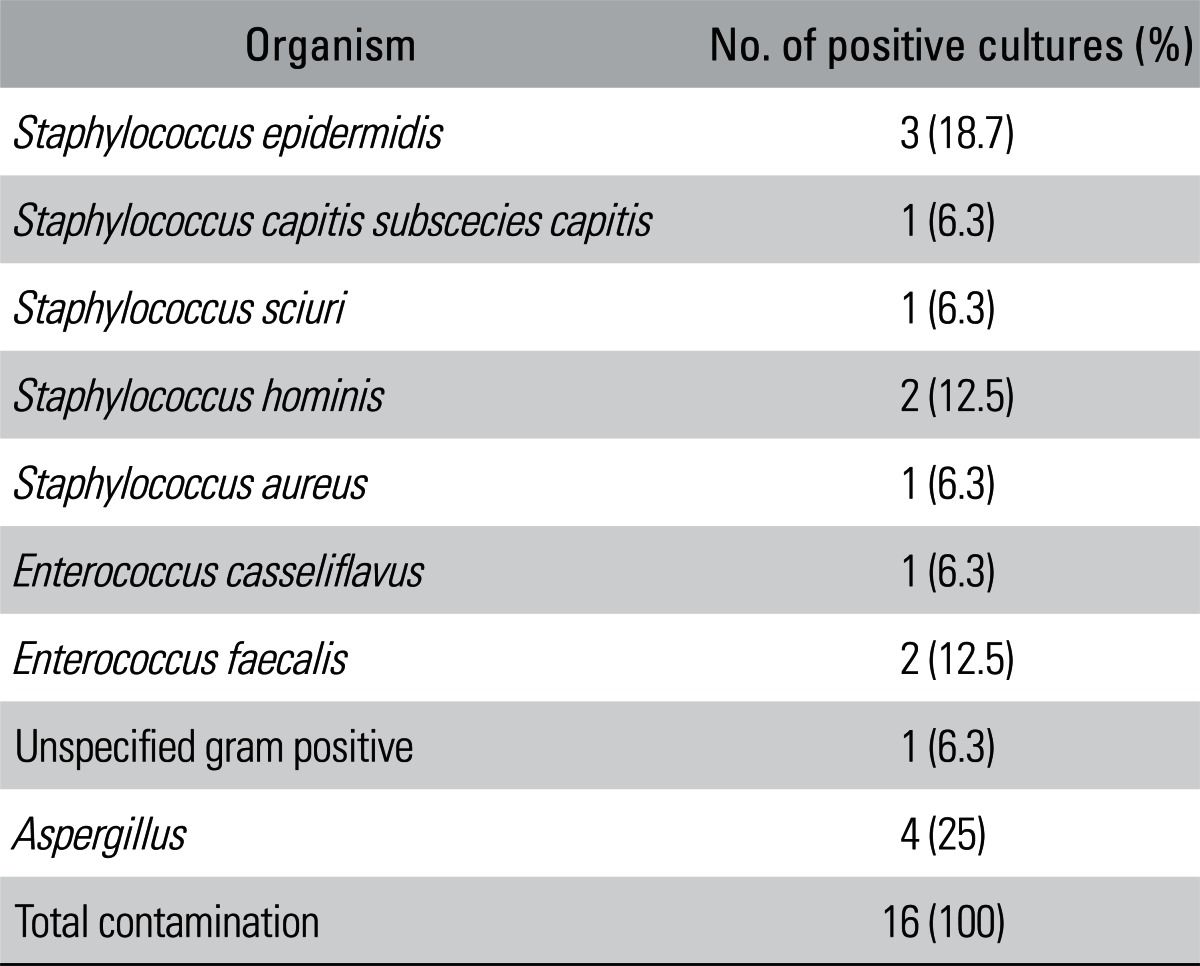
Of the 274 FHs, 16 (5.8%) returned positive microorganism cultures and were discarded from the bone bank. The remaining 258 FHs (94.2%) were available as donors. Single species of microorganism were identified in all 16 positive cultures. All bacterial contaminations of FHs were Gram-stain positive. The most common organism found was coagulase-negative staphylococci (7 cases), which accounted for 43.8% of the positive cultures. Enterococcus and Aspergillus were found in 3 cases (18.8%) and 4 cases (25%), respectively (Table 2). The rate of positive culture in the ON, OA, and FNF groups were 7.1%, 3.8%, and 4.0%, respectively. Although the positive FH rate was higher in the ON group than in the OA and FNF groups, this difference was not statistically significant (p > 0.05).
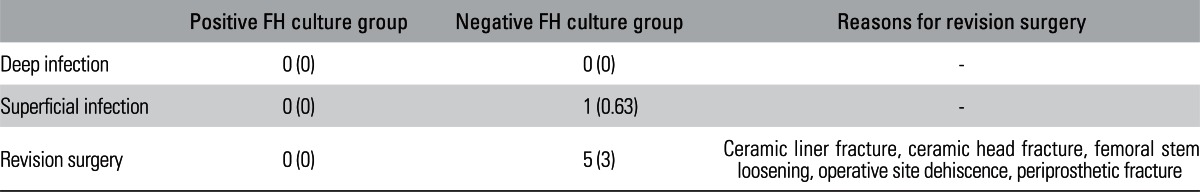
No postoperative infection occurred in any donor in the positive culture group, but one patient (0.63%) in the negative culture group experienced a postoperative superficial infection. This infection occurred early, and the donor had undergone a primary THR for OA. The FH from this patient was negative in all microbiological cultures. The culture of the purulent discharge from the superficial infection showed Staphylococcus aureus. The donor was treated with debridement and antibiotic therapy for two weeks.
During the follow-up of two years, 5 donors (3.2%) with aseptic FH underwent additional surgery for the following reasons: ceramic liner or head fracture, femoral stem loosening, operative site dehiscence and periprosthetic fracture. Of these five donors, three (1.8%) were in the ON group, one (1.3%) was in the OA group, and one (4%) was in the FNF group. No donor with a microbiologically contaminated FH underwent a revision surgery (Table 3).
In our hospital, the most common indication for primary THR is ON of the FH, followed by OA and FNF. Patient consent is obtained before the surgery regarding the donation of the excised FHs for the use in bone allografting, especially for the revision THR. Although bone allografting is useful for restoring a loss of bone during the revision THR, there are also risks of the recipient infection from the donated bones. Therefore the microbiological culture of the donated FHs is carried out, and the FHs with positive culture are discarded. In this study, the overall positive culture rate of 5.8% was similar to those reported by Salmela et al.8) in 2002 (5.2%) and van de Pol et al.9) in 2007 (6.4%). However, previous studies have reported the contamination rates as low as 1% to as high as 22%. We believe that this variability is explained by the different FH and hip joint sample numbers and the sampling locations. Furthermore, it has been reported that if only one single swab of the FH surface is tested, the number of false negatives increases.4,5) In our hospital, three samples are taken for each donated FH. These include a tissue sample of the cancellous bone and two swabs (the outer and inner FH surface). False negatives are not actively sought at our hospital, and the FHs are rinsed in the Ceftriaxone antibiotic solution and finally sterilized by gamma irradiation after the FH procurement.
Almost all organisms isolated in our study were commensal bacteria that normally resided on the skin and mucous membranes, such as coagulase-negative staphylococci, and in the human gastrointestinal tract, such as E. casseliflavus and E. faecalis. These bacteria have been commonly isolated from the FHs in other studies.4,9,10) In the present study, four FHs were contaminated with Aspergillus. Chazalet et al.11) found that the contamination with Aspergillus was associated with the peaks of the air and surface contamination at the hospital. Furthermore, in the present study, no donor with a positive FH culture developed a postoperative infection. Therefore, it is likely that the source of FH contamination in the cultures have originated from the environment and/or during the sampling process, rather than from a pre-existing infection.
As mentioned previously, ON of the FH is the leading cause of the primary THR at our hospital. Some reports have raised suspicions of a link between the concomitant ON of the FH and septic arthritis,12,13) and that ON of the FH makes it more prone to infection. The rationale for the development of ON of the FH is that the loss of the blood supply causes the devitalization and death of the bone tissues and provides favorable conditions for bacterial growth.14,15) Although it cannot be determined based on the microbiological culture findings whether an FH has been contaminated by handling and/or whether a positive finding truly reflects the presence of a preoperative infection, in the present study, the positive culture rate in the ON group was similar to those of the OA and FNF groups. ON of the FH may not advantage infection.
Only one of the 273 donors (0.58%) who underwent the primary THR experienced a postoperative infection. In this patient, S. aureus was found in a culture of the wound exudate, but the donor had an aseptic FH. In addition, no postoperative infection was encountered in donors with the contaminated FH. Our data shows that the rate of postoperative infections among the donors was low, and it was not related to the results of the microbiological cultures. The low infection rate of 0.63% in this study may be due to the good sterile conditions and antibiotic prophylaxis. The rate of culture-positive was not associated with the rate of postoperative infections. It may suggest that the contamination may happen while the FH samples are transported or in the processing of the microorganism culture. After THR, the donors with the positive FH were routinely administered intravenous antibiotics for 48 hours, and there was no infection. This suggests that the preventive antibiotics at induction of anaesthesia and within 48 hours after THR is effective in preventing infections. As there were no infections after THR even in the positive culture FHs, we think that the infection might have been within the FHs. It may also be that even with a mild degree of infection in the hip joint, the infection may be treated by removing the FH and joint cartilage or by the treatment with antibiotics. Other studies also have reported that a prolonged antibiotic prophylaxis after the operation does not decrease the surgical site infection.16,17) The rate of prosthetic failure in donors with the contaminated FH was no higher than in the aseptic group. These findings concur with those of other studies,4,6,7) but the previous studies did not include the analysis of the rates of the FH positive cultures, postoperative infections and prosthetic failures among the donors who underwent the primary THR for ON. The present study shows that the rates of the FH positive cultures, postoperative infections and prosthetic failures in the hips with ON were not higher than in the hips with OA or FNF. A further study of a larger group of patients is required to determine the nature of the association between ON and bone infection of FHs.
Our results suggest that there was no association between the microbiological culture results and the postoperative complications. Therefore, the microbiological culture results cannot be used as a prognostic factor of infection or prosthetic failure after primary THR. The FHs that return a positive culture may not lead to infection and/or other postoperative complication risks in the primary THR.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of the Department of Laboratory Medicine for the data. We would like to thank Nurse Bok Sik Kim for his kind help for the paper.
This study was supported by the grant CRI 12019-1 from the Chonnam National University Hospital Biomedical Research Institute.
References
1. Chapman PG, Villar RN. The bacteriology of bone allografts. J Bone Joint Surg Br. 1992; 74(3):398–399. PMID: 1587886.

2. Ivory JP, Thomas IH. Audit of a bone bank. J Bone Joint Surg Br. 1993; 75(3):355–357. PMID: 8496199.

3. Tomford WW, Ploetz JE, Mankin HJ. Bone allografts of femoral heads: procurement and storage. J Bone Joint Surg Am. 1986; 68(4):534–537. PMID: 3957976.
4. Sommerville SM, Johnson N, Bryce SL, Journeaux SF, Morgan DA. Contamination of banked femoral head allograft: incidence, bacteriology and donor follow up. Aust N Z J Surg. 2000; 70(7):480–484. PMID: 10901573.

5. Saegeman V, Verhaegen J, Simon JP. Screening femoral heads from living donors: a prospective study comparing swab-agar versus bone fragment-broth culture. Acta Orthop Belg. 2011; 77(3):381–385. PMID: 21846008.
6. James LA, Gower A. The clinical significance of femoral head culture results in donors after hip arthroplasty: a preliminary report. J Arthroplasty. 2002; 17(3):355–358. PMID: 11938514.
7. James LA, Ibrahim T, Esler CN. Microbiological culture results for the femoral head. Are they important to the donor? J Bone Joint Surg Br. 2004; 86(6):797–800. PMID: 15330017.
8. Salmela PM, Hirn MY, Vuento RE. The real contamination of femoral head allografts washed with pulse lavage. Acta Orthop Scand. 2002; 73(3):317–320. PMID: 12143980.

9. van de Pol GJ, Sturm PD, van Loon CJ, Verhagen C, Schreurs BW. Microbiological cultures of allografts of the femoral head just before transplantation. J Bone Joint Surg Br. 2007; 89(9):1225–1228. PMID: 17905962.

10. Journeaux SF, Johnson N, Bryce SL, Friedman SJ, Sommerville SM, Morgan DA. Bacterial contamination rates during bone allograft retrieval. J Arthroplasty. 1999; 14(6):677–681. PMID: 10512439.

11. Chazalet V, Debeaupuis JP, Sarfati J, et al. Molecular typing of environmental and patient isolates of Aspergillus fumigatus from various hospital settings. J Clin Microbiol. 1998; 36(6):1494–1500. PMID: 9620367.
12. Hernigou P, Daltro G, Flouzat-Lachaniette CH, Roussignol X, Poignard A. Septic arthritis in adults with sickle cell disease often is associated with osteomyelitis or osteonecrosis. Clin Orthop Relat Res. 2010; 468(6):1676–1681. PMID: 19885711.

13. Lee YK, Lee YJ, Ha YC, Kim KC, Koo KH. Septic arthritis of the hip in patients with femoral head osteonecrosis. Arch Orthop Trauma Surg. 2011; 131(11):1585–1590. PMID: 21660480.

14. Blacksin MF, Finzel KC, Benevenia J. Osteomyelitis originating in and around bone infarcts: giant sequestrum phenomena. AJR Am J Roentgenol. 2001; 176(2):387–391. PMID: 11159079.
15. Poignard A, Bouhou M, Homma Y, Hernigou P. Septic arthritis of the hip in adults with sickle cell anemia. Orthop Rev (Pavia). 2011; 3(1):e1. PMID: 21808713.
16. van Kasteren ME, Mannien J, Ott A, Kullberg BJ, de Boer AS, Gyssens IC. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin Infect Dis. 2007; 44(7):921–927. PMID: 17342642.

17. Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000; 101(25):2916–2921. PMID: 10869263.

Table 1
Donor Selection Criteria
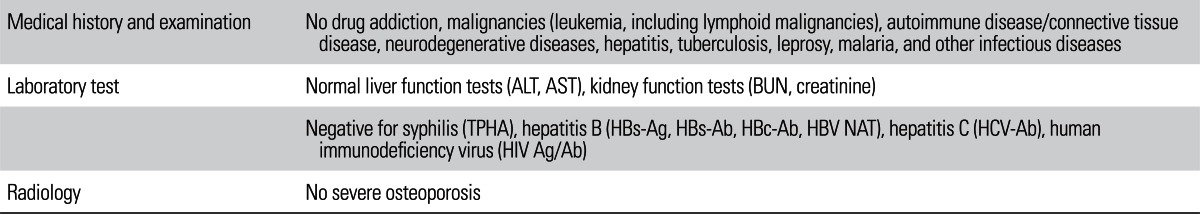
ALT: alanine transaminase, AST: aspartate transaminase, BUN: blood urea nitrogen, TPHA: treponema pallidum hemagglutination, HBs-Ag: hepatitis B surface antigen, HBs-Ab: hepatitis B surface antibody, HBc-Ab: hepatitis B core antibody, HBV NAT: hepatitis B virus nucleic acid testing, HCV-Ab: hepatitis C virus antibody, HIV Ag/Ab: human immunodeficiency virus antigen/antibody.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download