Abstract
Background
To determine the prevalence of spondylolysis in a selected population and evaluate the association of spondylolysis with low back pain (LBP). Spondylolysis is widespread in the general population but the prevalence of spondylolysis and its relationship with LBP in the Korean population is controversial.
Methods
A sample of 855 participants (age, 20 to 86 years) from our medical center who underwent multidetector computed tomography (CT) imaging to assess abdominal and urological lesions were enrolled in this study. The occurrence of LBP requiring medication in the preceding 12 months was evaluated using a self-report questionnaire (a modified Nordic Low Back Pain Questionnaire). The presence of spondylolysis was characterized by CT imaging. Multiple logistic regression models were used to examine the association between spondylolysis and LBP, while adjusting for gender and age.
Results
Seventy-eight study subjects (9%) demonstrated spondylolysis on CT imaging. There was no significant difference between the age groups (p = 0.177). The p-value of gender was 0.033 but this was not significant due to the selected population bias. Three hundred eleven study subjects (36%) had back pain. There was a significant difference between gender (p = 0.001). No significant association was identified between spondylolysis and the occurrence of LBP.
Conclusions
The prevalence of LBP was 36.37% and the prevalence of lumbar spondylolysis in a selected population, who visited hospital for abdominal or urological lesions except LBP, was 9.12% based on CT imaging. Males demonstrated a similar presence of LBP to females but a significantly higher incidence of spondylolysis (p = 0.033). The prevalence of spondylolysis was not associated with the presence of LBP and age in adulthood.
Lumbar spondylolysis refers to the dissolution of, or a defect in, the pars interarticularis of a vertebra, which is most commonly observed in the lowest lumbar vertebrae. Lumbar spondylolysis is often identified in the course of clinically evaluating patients with low back pain (LBP), and it has been estimated that 25% of individuals with spondylolysis experience at least 1 episode of significant back pain at some point in their lifetime.
The relationship between spondylolysis and clinically significant LBP has been a subject of ongoing controversy. The prevalence of a defect in the pars interarticularis is approximately 5% in the general population in the USA1) but this varies from study to study. A recent examination using lateral plain film radiographs suggest that lumbar spondylolysis can develop in adulthood.2) However, the use of plain radiographs in these studies is less sensitive for detecting unilateral or early non-slipped defects.3) Computed tomography (CT) is currently considered the most accurate imaging modality for identifying spondylolysis and it often reveals the presence of non-displaced spondylolysis.3,4) Although spondylolysis has been well studied in selected symptomatic patient populations, i.e. patients presenting to a clinic for the treatment of LBP, few studies have demonstrated its significance in adults. The aims of this study were 1) to evaluate the prevalence of spondylolysis in different age groups and according to gender in a selected population who visited hospital for reasons other than LBP, and 2) to evaluate the association of spondylolysis with LBP in the same population cohort.
The research protocol for this study was approved by the institutional review board. This retrospective study included patients who had undergone CT between January 1st 2009 and December 31st 2009. Patients who met the following criteria were selected: over the age of 20 years, provided informed consent and underwent CT examinations to assess abdominal or urological lesion for reasons unrelated to LBP. All the CT scans ordered from the Departments of General Surgery and Urology were included, and the CT scans which had been checked for a LBP evaluation from any related department, such as Orthopaedic Surgery, Neurologic Surgery, and Rehabilitation, were excluded. To prevent result bias, patients with LBP as the primary indication for the CT examination were excluded.
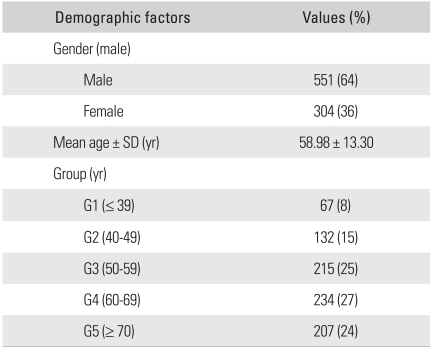
A total of 855 participants, aged from 20 to 86 years, were enrolled consecutively and assessed for the association between the CT-observed spondylolytic characteristics of the lumbosacral spine and LBP. The patients were grouped into 5 groups according to age (group 1, below 39 years; group 2, 40 to 49 years; group 3, 50 to 59 years; group 4, 60 to 69 years; and group 5, above 70 years).
All participants, who had undergone multidetector CT scanning, were asked to complete the modified Nordic Low Back Pain Questionnaire,5) which was administered by senior trained nurses. The first question on the questionnaire that was translated into Korean was: "Have you suffered low back pain almost every day for at least 1 month over the last 12 months?" The individuals' answers of "yes" or "no" to the above question were used in this study as the back pain outcome. Similar methods have been used by several authors6,7) for work-related compensation assessments.
CT was performed on one of three 16-multidetector computed tomography (MDCT) machines or a dual source 64-MDCT system (Lightspeed Ultra, GE, Milwaukee, WI, USA). Because the images were obtained from patients with different indications and using different protocols, the axial slice thickness was varied from 0.75 mm to 5 mm.
All the CT scans were analyzed in a blinded manner with respect to the clinical and personal data. All the CT scans were reviewed by two orthopedic surgeons (SBK, an orthopedic spine surgeon with 8 years experience, and BRH, a certified orthopedic surgeon with 1 year experience). The images were reviewed on a secure-access picture-archiving communication system (PACS; Philips Spectra, Eindhoven, The Netherlands). All the CT images that had been initially reviewed were axial images. All examinations were evaluated to determine the existence of linear lucidity or a defect extending through the pars interarticularis in the lower lumbar spine and whether the defect was unilateral or bilateral.
The prevalence of spondylolysis in the 5 different age groups and according to gender was calculated. Those prevalence estimations were compared using a χ2 test or Fisher's exact test. Multiple logistic regression analysis was used to examine the association between LBP and spondylolysis while adjusting for gender and age. The prevalence of those studied conditions in the subjects with and without LBP was also compared. All statistical analyses were performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered significant.
The 855 subjects included 551 men (64%) and 304 women (36%). The mean age was 58.98 ± 13.30 years (range, 20 to 86 years). Tables 1 and 2 provide further details of the demographic characteristics of the subjects and detailed descriptive statistical data of this study, respectively.
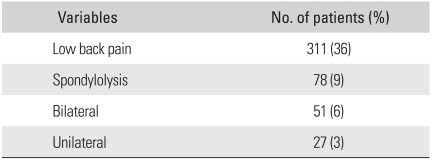
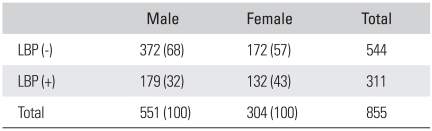
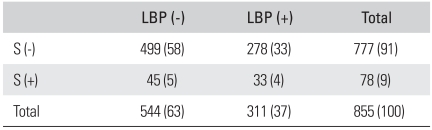
Within this population, 311 patients (36%) had LBP. Among the populations presenting with LBP, 179 (21%) subjects were male and 132 (15%) subjects were female. Table 3 lists the presence of LBP in each gender. Men demonstrated a significant greater presence of LBP than women (p = 0.001). There was no significant association between the presence of LBP and age.
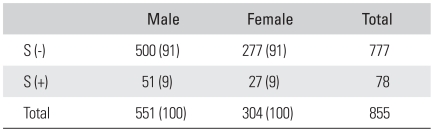
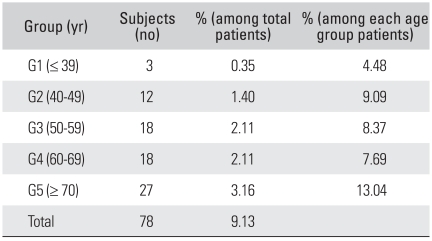
Out of 78 spondylolysis patients (9%), 51 (65%) were male and 27 (35%) were female. Men demonstrated a significantly higher prevalence of spondylolysis (p = 0.033) but this difference was not significant due to the selected population bias. Table 4 lists the number of subjects and the percentages among the spondylolysis patients. Table 5 presents the prevalence of spondylolysis according to age group. The prevalence of spondylolysis was similar in the age groups (p = 0.177). Fifty one patients (6% of the study population and 65% of the spondylolysis population) demonstrated bilateral spondylolysis and 27 patients (3% of the study population and 35% of the spondylolysis population) demonstrated unilateral spondylolysis.
Seventy eight patients (9% of the study population) demonstrated lumbar spondylolysis on the CT findings. Within this spondylolysis population, 33 patients (42%) had back pain and 45 patients (58%) did not. Table 6 compares the prevalence of spondylolysis in the groups of individuals with and without LBP. The prevalence of spondylolysis was not associated with the occurrence of LBP. Table 6 lists the results of multiple logistic regression analysis where LBP was a dependent variable and the presence of spondylolysis in the different gender and age groups was included as an independent variable. There were no significant associations found between spondylolysis and the aforementioned predicting variables (p > 0.05 for each association).
There are few reports that have focused on the incidence of spondylolysis in Asian populations, such as Japanese and Koreans, the reports that are available have some weaknesses. One is that the subjects of the investigation were not part of the general population but were LBP patients. Another weakness lies in the methods used in the analysis, such as plain radiographs or skeletal investigation. Despite these potential pitfalls such as the representative Korean population in this study, the strength of this study is that a selected community sample was included, multidetector CT scans were used to detect spondylolysis and the younger age group (below 20 years old) was excluded. This CT imaging modality is currently considered the gold standard for identifying spondylolysis.
Hu et al.1) reported that the prevalence of a defect in the pars interarticularis was approximately 5% in the general population. Some authors8-10) reported that the prevalence of spondylolysis was 6% in adults. The previous large screening study of Virta et al.,10) which is often cited, was based only on plain radiographs. Of course, ethnic variations are a possible contributory factor. For example, the Native American and Eskimo population has a very high incidence of spondylolysis, ranging from 17% to 53%9) and the prevalence of spondylolysis in the Caucasian population is two to three times higher than that in the African population.8,9) Kalichman et al.9) reported that the prevalence of lumbar spondylolysis in an unselected community-based population is 11.5%, which is almost twice the prevalence of the previous plain radiograph-based studies. Similar to this study, a likely explanation for the significantly higher rate identified in the current study is the use of CT. CT is a highly advanced imaging modality and is considered the gold standard for identifying spondylolysis, particularly the unilateral and non-displaced bilateral cases. Sakai et al.,11) who used CT imaging on 2000 subjects, reported a 5.9% incidence of spondylolysis in a Japanese population. The incidence of spondylolysis of this study was 9.12%, which is similar to Kalichman et al.9) but much higher than Sakai et al.11)
In terms of the epidemiologic patterns, there was no significant difference in the prevalence of spondylolysis between men and women. However, the male-to-female ratio was almost 2:1 in previous studies.1,11-13) Waldron14) suggested in 1991 that the difference in incidence between genders may be a rather recent phenomenon. The reason for the difference has yet to be clarified.
In this study, no significant associations were found between spondylolysis and the presence of LBP (p = 0.402). The relationship between spondylolysis and the occurrence of LBP is controversial.8,15) A recent study by Miyauchi et al.16) reported that spondylolysis appeared as pseudoarthrosis, and there was no histological correlation with chronic LBP. Individuals engaged in specific athletic activities appeared more likely to develop symptomatic LBP associated with spondylolysis.17) The pathologic mobility of the "Gill fragment" of the spinal lamina is considered to be a LBP source but in many cases, spondylolysis is found incidentally in the asymptomatic general population.8,15)
No significant associations were found between spondylolysis and adult age. Hu et al.1) stated that the prevalence of spondylolysis was 4.4% at six years of age and 6% in adulthood. Eisenstein18) reported that only 1 of the 485 skeletons he examined had unilateral spondylolysis. Sakai et al.11) showed that the ratio of unilateral spondylolysis to bilateral spondylolysis is 21.0%. However, in our study, 27 of a total 78 spondylolytic subjects (34.6%) had unilateral spondylolysis. It is not possible to conclude that the ratio of unilateral spondylolysis to all spondylolysis is more frequent in Koreans according to the current data. In addition, the displacement of spondylolysis was not evaluated on a CT axial image of abdomen or pelvis. Hence, the relationship between LBP and the displacement of spondylolysis also was not known.
In conclusion, the incidence of lumbar spondylolysis in the 855 adults in this study was 78 subjects, i.e., 9% (males, 6%; females, 3%). The male-to-female ratio in this study was -1:1 for spondylolysis. No significant association was found between the presence of spondylolysis on CT and the occurrence of LBP. This suggests that the condition does not represent a major cause of LBP in the general population.
References
1. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008; 90(3):656–671. PMID: 18326106.
2. Sonne-Holm S, Jacobsen S, Rovsing HC, Monrad H, Gebuhr P. Lumbar spondylolysis: a life long dynamic condition? A cross sectional survey of 4.151 adults. Eur Spine J. 2007; 16(6):821–828. PMID: 17120072.

3. Teplick JG, Laffey PA, Berman A, Haskin ME. Diagnosis and evaluation of spondylolisthesis and/or spondylolysis on axial CT. AJNR Am J Neuroradiol. 1986; 7(3):479–491. PMID: 3085451.
4. Krupski W, Majcher P, Tatara MR. Computed tomorgaphy diagnostic of lumbar spondylolysis. Ortop Traumatol Rehabil. 2004; 6(5):652–657. PMID: 17618216.
5. Kuorinka I, Jonsson B, Kilbom A, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987; 18(3):233–237. PMID: 15676628.

6. Dovrat E, Katz-Leurer M. Cold exposure and low back pain in store workers in Israel. Am J Ind Med. 2007; 50(8):626–631. PMID: 17595006.

7. Ghaffari M, Alipour A, Jensen I, Farshad AA, Vingard E. Low back pain among Iranian industrial workers. Occup Med (Lond). 2006; 56(7):455–460. PMID: 16837536.

8. Fredrickson BE, Baker D, McHolick WJ, Yuan HA, Lubicky JP. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984; 66(5):699–707. PMID: 6373773.

9. Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine (Phila Pa 1976). 2009; 34(2):199–205. PMID: 19139672.
10. Virta L, Ronnemaa T, Osterman K, Aalto T, Laakso M. Prevalence of isthmic lumbar spondylolisthesis in middle-aged subjects from eastern and western Finland. J Clin Epidemiol. 1992; 45(8):917–922. PMID: 1624974.

11. Sakai T, Sairyo K, Takao S, Nishitani H, Yasui N. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine (Phila Pa 1976). 2009; 34(21):2346–2350. PMID: 19934813.

12. Newman PH. Stenosis of the lumbar spine in spondylolisthesis. Clin Orthop Relat Res. 1976; (115):116–121. PMID: 1253474.

13. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976). 2003; 28(10):1027–1035. PMID: 12768144.
14. Waldron HA. Variations in the prevalence of spondylolysis in early British populations. J R Soc Med. 1991; 84(9):547–549. PMID: 1941859.

15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine (Phila Pa 1976). 2003; 28(3):255–259. PMID: 12567027.
16. Miyauchi A, Baba I, Sumida T, Manabe H, Hayashi Y, Ochi M. Relationship between the histological findings of spondylolytic tissue, instability of the loose lamina, and low back pain. Spine (Phila Pa 1976). 2008; 33(6):687–693. PMID: 18344864.

17. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993; 6(5):406–411. PMID: 8274809.
18. Eisenstein S. Spondylolysis. A skeletal investigation of two population groups. J Bone Joint Surg Br. 1978; 60(4):488–494. PMID: 361744.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download