Abstract
Background
Scar tissue formation is the major cause of failure in peripheral nerve surgery. Use of a hyaluronic acid-carboxymethylcellulose (HA-CMC) membrane (Seprafilm) as a solid anti-adhesion barrier agent is one of the therapeutic approaches to reduce postoperative scar tissue formation. However, a solid membrane may not be suitable for repair of a weak peripheral nerve site. This study examined the effect of HA-CMC solution on perineural scar formation after peripheral nerve repair in rats.
Methods
The sciatic nerves of 40 rats were transected and then immediately repaired using 10-0 nylon. The nerves were divided randomly into two groups. Saline and HA-CMC solution were applied topically to the nerve repair sites in the control and experimental groups, respectively. Reoperation was performed at 3, 6, 9, and 12 weeks to assess scar tissue formation. The assessment included the quality of wound healing, presence of perinueral adhesion, cellular components of the scar tissue, thickness of the scar tissue and histomorphological organization of the repair site.
Results
Topical application of the HA-CMC solution significantly decreased the macroscopic nerve adherence score and the numbers of the cellular components such as fibroblasts and inflammatory cells (p < 0.05, Mann-Whitney U-test). The scar tissue formation index was significantly lower in the experimental group at 12 weeks than that in the control group (p < 0.05, Mann-Whitney U-test). The grading scores of the histomorphological axonal organization at the repair site were significantly higher in the experimental group than those in the control group at 12 weeks (p < 0.05, Mann-Whitney U-test). No evidence of wound dehiscence or inflammatory reactions against the HA-CMC solution was noted.
Functional recovery after peripheral nerve injuries with laceration is unsatisfactory even if these injuries are immediately repaired using microsurgical procedures. Several factors are responsible for incomplete recovery. One of the main causes of failure is scar tissue formation of the injured nerve.1,2) Intraneural scarring may develop due to intraoperative neural injury, hemorrhage in the surgical field and even simple manipulation of the nerve. Intraneural scarring acts as a mechanical barrier against axonal regeneration and it disturbs nerve conduction.1,2) Extraneural scarring may lead to chronic compression or tethering of the nerve to the surrounding tissue, which can cause traction injury and vasospasm of the intraneural vessel. Consequently, this may lead to ischemia of the nerve and irreversible nerve injury.1,2) Intraneural and extraneural scarring and perineural adhesion of nerves may induce chronic persistent neurologic symptoms. Thus, adhesion has become an important complication of peripheral nerve surgery.
Hyaluronic acid (HA) is a natural component of the extracellular matrix, and it plays an important role with the hyaluronate-fibrin matrix in wound healing.3) Hyaluronic acid reduces perineural scarring and postoperative adhesion, and improves peripheral nerve regeneration.4-8) Carboxymethylcellulose (CMC) is a biocompatible polysaccharide that acts as a physical barrier and can reduce peripheral nerve adhesion and epidural fibrosis after spine surgery.9-11) Mixing hyaluronic acid with carboxymethylcellulose not only delays the absorption of HA, but also prolongs the duration of the anti-adhesion effect of HA.9,10)
Seprafilm is a commercially-available HA-CMC film-type solid membrane (Genzyme, Cambridge, MA, USA) that retards adhesion. The demonstration of the prowess of Seprafilm for the prevention of abdominal adhesion after surgery12) has led to the widespread use of the HA-CMC membrane experimentally as well as clinically to prevent adhesion in abdominal surgeries, pelvic surgeries, tendon surgery in the hand and spinal surgery. However, there have been only a few reports on the use of the HA-CMC membrane for the prevention of peripheral nerve adhesion.13,14) The solid, film-like HA-CMC membrane is relatively rigid compared to nerves, which confers some physical restrictions on its use with nerves.7,15)
More recently, a solution form of HA-CMC has been developed (Guardix-sol; Biorane, Seoul, Korea). The solution form is chemically identical to the HA-CMC membrane and is more suitable and easily-applied to a weak peripheral nerve than the solid film type. Until now, there have been no reports on the prevention of the adhesion of peripheral nerves using the HA-CMC solution. The purpose of this study was to evaluate the effect of HA-CMC solution on the formation of perineural scar after repair of the sciatic nerve in a rat model.
All the procedures for the care and use of laboratory animals were conducted in accordance with the guidelines outlined by the Experimental Animal Ethics Committee of Kyung Hee University. Forty adult Sprague-Dawley rats (Sam:TacN(SD)Br; Samtako, Seoul, Korea) each weighing 300-400 g were used. Standard rat laboratory food and water were supplied ad libitum. During the experiment, two rats died and two other rats were added as replacements. All the animals were adjusted to the laboratory environment by keeping them in an animal room for 2 weeks at constantly maintained temperature and humidity. The rats were randomly divided into the control group (n = 20) and the experimental group (n = 20).
The rats were anesthetized with an intraperitoneal injection of 1 mg/kg tiletamine HCl/Zolazepam HCl (Zoletil; Virbac, Carros, France). If the anesthesia was not sufficient, the same drug at a dose of 0.5 mg/kg was additionally injected. Following hair shaving and disinfection with betadine solution, the left sciatic nerve of each rat was exposed through a gluteal muscle-splitting approach. Under microsurgical dissection, the sciatic nerve was sharply transected and then the epineurium was immediately repaired using Ethilon 10-0 monofilament polyamide nylon (Ethicon; Johnson & Johnson, Somerville, NJ, USA). In the experimental group, 1 mL of Guardix-sol was applied around the nerve including the repair site. In the control group, 1 mL of normal saline was applied around the nerve including the repair site. The muscle fascia and the skin were closed in layers. The rats were allowed unrestricted movement without any immobilization after recovery from the anesthesia. Sufficient animal food and water were provided, and prophylactic antibiotics were administered until postoperative day 2.
After the macroscopic evaluation, all the rats were euthanized by an overdose of anesthetic agents. The entire sciatic nerve and surrounding tissue, including the repaired site, was removed en bloc. The specimens were fixed in 10% neutral formalin and then embedded in paraffin. Five micrometer-thick, longitudinal serial sections were obtained from the specimen. They were stained with hematoxylin and eosin (H&E) for the cellular components and with Masson's trichrome for the collagen within the scar tissue. Also, the H&E-stained sections were used to assess the histomorphological organization of the nerve repair site. All the histological evaluations were performed blindly by a pathologist.
The cellular components were analyzed to determine the number of fibroblasts and inflammatory cells around the nerve repair site. The numbers of fibroblasts and inflammatory cells were counted from four different quadrants of the perineural scar tissue around the repair site in each nerve at × 400 magnification (Fig. 1). The numbers (mean ± standard deviation) of fibroblasts and inflammatory cells in each group were calculated.
The dense scar tissues surrounding the nerve were distinguishable as a dark-staining longitudinal band with using Masson's trichrome. For the quantitative analysis of the scar tissues, the thickness of the thickest area of scar tissue was measured using a microscope, and this was divided by the thickness of the nerves in the same area to obtain the standardized ratio. The value obtained represented the scar tissue formation index (Fig. 2). At each experimental week, the differences of the average of the scar tissue formation index of the control group and the experiment group were compared and analyzed.
The longitudinal organization and morphology of the axon at the nerve repair site was evaluated. The evaluation was performed according to a previously-described scale17) (1: failure, no continuity of the axons from the proximal to distal ends; 2: poor organization of the repair site; 3: fair organization of the repair site; 4: good organization of the repair site, approaching normal; 5: excellent organization of the repair site, indistinguishable from normal) (Table 2). At each week, the scores of the control group and the experimental group were compared and analyzed.
The statistical analysis was conducted using IBM SPSS ver. 18.0 (IBM, New York, NY, USA). The mean ± standard deviation of each set of data were obtained, and those for the control group and the experimental group were compared by the non-parametric Mann-Whitney U-test. Values were considered statistically significant when the p-value was < 0.05.
The statuses of the skin and the fascia were good in both groups. There was no sign of infection or an inflammatory reaction. The nerves treated with saline demonstrated dense scar tissue formation surrounding the repair site, whereas the nerves treated with HA-CMC solution demonstrated a thin, lucent membrane-like tissue surrounding the repair site. The perineural adhesion in the experimental group was statistically significantly lower than that in the control group (p < 0.05) (Fig. 3).
At 3 weeks after surgery, the nerves treated with saline demonstrated a moderate infiltration of inflammatory cells and fibroblasts at the nerve repair site. However, the nerves treated with HA-CMC solution demonstrated a mild infiltration of inflammatory cells and fibroblasts. At 6, 9, and 12 weeks after surgery, there was a significant reduction of the cellular components in the experiment group as compared with the control group. The number of fibroblasts in the scar tissues of the experimental group at all the time periods was significantly less than that of the control group (p < 0.05) (Fig. 4). Similarly, the number of inflammatory cells of the experimental group was significantly less than that of the control group at all the time periods (p < 0.05) (Fig. 5).
At 3 weeks after surgery, the thickness of the scar tissue in the nerves treated with HA-CMC solution seemed to be similar with that in the nerves treated with saline (Fig. 6B and 6D). However, the thickness of the scar tissues in the nerves treated with saline was more increased than that in the nerves treated with HA-CMC solution at 6 weeks (Fig. 7B and 7D). At 9 and 12 weeks, there seemed to be an increased thickness of the scar tissue in the epineurium of the nerves treated with saline, as compared with that of the nerves treated with HA-CMC solution (Figs. 8B, 8D, 9B, and 9D). However, the scar tissue formation index in the experimental group was significantly lower than that in the control group only at 12 weeks (p < 0.05) (Fig. 10).
At 3 weeks after surgery, the continuity of the nerve repair site was good in both groups, and the organization at the repair site, including the alignment of axons, was similar between each group (Fig. 6A and 6C). At 6 and 9 weeks after surgery, the axonal alignments in the nerves treated with HA-CMC solution demonstrated a focal whirling appearance, but they were mostly more parallel than those in the nerves treated with saline (Figs. 7A, 7C, 8A, and 8C). On examination at 12 weeks after surgery, the organization at the nerve repair site in the nerves treated with HA-CMC solution was significantly better than that in the nerves treated with saline (Fig. 9A and 9C, p < 0.05; Fig. 11).
There have been many attempts to reduce epineurial scarring and perineural adhesion after peripheral nerve surgery. There have been various surgical techniques to prevent perineural scarring and adhesion, such as the microsurgical technique, flap,18) free fat graft,19) vein wrapping20) and silicone cuffing.21) Postoperative perineural scarring and adhesion are inevitable, even when using meticulous surgical techniques, including microsurgical techniques. Several pharmacologic agents reduce scar formation in peripheral nerve surgery, including cis-hydroxyproline,22) anti-transforming growth factor-β1 antibody,23) citicoline,24) ADCON-T/N,16) and doxorubicin.25) All these drugs may have positive effects in experimental studies, but they have not yet been applied clinically.
Other pharmacologic agents for reducing scar formation are HA and its derivates, which have the effect of optimizing the extracellular matrix.13,14) HA is a major component of the extracellular matrix and it plays an important role in the early wound healing process.3) HA is an endogenous stimulator of interleukin-1 (IL-1) production and IL-1 affects fibroblast proliferation and collagenase production.26) HA also regulates leukocyte motility, adhesion and phagocytosis, and so it suppresses the scar formation process caused by the infiltration of inflammatory cells to damaged tissues.27) HA and its derivatives have been utilized as a surgical coat, as a physical barrier that prevents the adhesion of adjacent tissues with the nerve.7,15,28) Coating the surgical field with HA from the beginning to the end of surgery can reduce scar formation.7) It has been reported that HA-CMC membrane does not affect expression of transforming growth factor-beta 1, type 1 collagen, matrix metalloproteinase (MMP)-1, MMP-2, tissue inhibitor of metalloproteinase 1 and tissue plasminogen activator (tPA), but does act as a physical barrier against adhesion.29) The HA derivative Hyaloglide (an auto-cross-linked polysaccharide gel; Fidia Advanced Biopolymers, Abano Terme, Italy) has been demonstrated to significantly decrease perineural adhesion after peripheral nerve surgery at 4 weeks after surgery.28) Consistent with this, the present study found that the number of inflammatory cells and fibroblasts in the experiment group were significantly lower than that in the control group (p < 0.05).
As reported by previous studies, HA and its derivatives may also promote regeneration of injured nerves through the realignment of the fibrin matrix and they can provide a suitable environment for axonal ingrowth. In one study, repeated injection of HA using nerve conduits enhanced nerve regeneration in the sciatic nerve defects of rats.4) In another study, HA reduced perineural adhesion 4 weeks and 12 weeks after neurorrhaphy, and enhanced peripheral nerve regeneration at 12 weeks after surgery.8) Other authors reported that the HA-CMC membrane had favorable effects on reducing extraneual adhesion and promoting nerve regeneration in rabbits at 3 months after sciatic nerve repair.13) However, contrarily, another study reported that Seprafilm had no deleterious effects on the nerve repair site, and it had favorable effects on perinueral scar and adhesion formation.14) The latter authors suggested that the HA-CMC membrane was not effective for nerve regeneration. The results of still another study indicated that the application of Hyaloglide did not significantly increase the regenerated axonal counts at 4 weeks after nerve repair.28)
Anti-adhesion agents comprise various types according to their physical characteristics: liquid, gel and membrane. Products containing a HA-CMC component consist of a solution type and a membrane type. Seprafilm is a solid membrane type agent, which may not be suitable for use in special situations. Peripheral nerves may be too weak and fragile to surround with a solid film-type agent.7) In contrast, solution-type agents are easy to use and are free from restrictions relating to shape or extent.15) We absolutely agree with the view that a peripheral nerve repair site is very fragile and thin. Additionally, there has not been any report on the effects of HA-CMC solution on extraneural scarring and adhesion and peripheral nerve regeneration.
In this study, macroscopic evaluation demonstrated that the adhesion of nerves to adjacent tissues was significantly reduced in the experimental group as compared with that of the control group at weekly intervals (p < 0.05). Histological analysis determined that the scar tissue formation index of the experimental group was significantly lower than that of the control group at 12 weeks after surgery (p < 0.05). Histomorphological analysis of the organization of the nerve repair sites revealed that the experiment group was significantly better than that of the control group at 12 weeks after surgery (p < 0.05).
Additionally, there have been some reports about the complications of anti-adhesion agents, including deterioration of wound healing and an inflammatory reaction. It has been reported that ADCON-T/N, a carbohydrate polymer gel, is effective for reducing perineural adhesion after peripheral nerve surgery, yet it may have a deleterious effect on wound healing.16,28) Further, HA-CMC membrane (Seprafilm) may cause severe inflammatory reactions after surgery.30) However, other studies have reported that HA and its derivatives are safe and do not have deleterious effects on wound healing.7,8,13,14) In this study, there was no difference in wound healing between the experimental group and the control group.
In conclusion, local application of HA-CMC solution had favorable effects on extraneural scar formation and adhesion after peripheral nerve repair. It also enhanced the organization of the nerve repair site, which is one of the indicators of nerve regeneration. A functional analysis is needed to clarify the anti-adhesion effect and nerve regeneration effects of HA-CMC solution, and a comparative analysis between HA-CMC membrane and HA-CMC solution is needed to determine the benefits related to the physical characteristics of the HA-CMC agents.
Figures and Tables
Fig. 1
Schematic diagram of the assessment of the cellular components in the scar tissue. The mean value of the cellular components of each quadrant in the extraneural scar tissue was calculated at × 400 magnification.
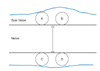
Fig. 2
Schematic diagram of the scar tissue formation index. The scar tissue with the largest thickness was normalized by dividing it by the nerve diameter. Scar tissue formation index = the ratio of the thickness of the extraneural scar tissue to the nerve diameter (a/b).
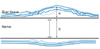
Fig. 3
Results of the macroscopic assessment of nerve adherence. The scores of the experimental group were significantly lower than the scores of the control group (*p < 0.05).

Fig. 4
Results of the fibroblast counts. The fibroblast counts of the
experimental group were significantly lower than the fibroblast counts of
the control group (*p < 0.05).

Fig. 5
Results of the inflammatory cell counts. The inflammatory cell counts of the experimental group were significantly lower than the inflammatory cell counts of the control group (*p < 0.05).

Fig. 6
Photomicrographs showing the longitudinal sections of the sciatic nerves following cut and repair with saline (A, B) and with the hyaluronic acid-carboxymethylcellulose (HA-CMC) solution (C, D) at 3 weeks after surgery. The sections were stained with H&E (A, C) and with Masson's trichrome (B, D). Original magnification was × 40. In the nerves treated with saline, a moderate inflammatory cell infiltration and the interlacing or whirling appearance of axons were demonstrated at the repair site (A). In the nerves treated with the HA-CMC solution, a mild inflammatory cell infiltration and the whirling and focal parallel appearance of axons were demonstrated at the repair site (C). The thickness of the blue-stained collagen depositions surrounding the nerve in the saline group was similar to that in the HA-CMC solution group (B, D).
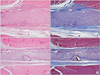
Fig. 7
Photomicrographs showing the longitudinal sections of the sciatic nerves following cut and repair with saline (A, B) and with the hyaluronic acid-carboxymethylcellulose (HA-CMC) solution (C, D) at 6 weeks after surgery. The sections were stained with H&E (A, C) and with Masson's trichrome (B, D). Original magnification was × 40. In the nerves treated with saline, a moderate inflammatory cell infiltration and an interlacing or whirling appearance of axons were demonstrated at the repair site (A). However, in the nerves treated with HA-CMC solution, a mild inflammatory cell infiltration and a relatively parallel appearance of axons were demonstrated at the repair site (C). The thickness of the blue-stained collagen depositions surrounding the nerve in the saline group was thicker than that in the HA-CMC solution group (B, D).
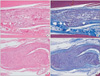
Fig. 8
Photomicrographs showing the longitudinal sections of the sciatic nerves following cut and repair with saline (A, B) and with the hyaluronic acid-carboxymethylcellulose (HA-CMC) solution (C, D) at 9 weeks after surgery. The sections were stained with H&E (A, C) and with Masson's trichrome (B, D). Original magnification was × 40. In the nerves treated with saline, the relatively parallel appearance of axons with focal interlacing was observed at the repair site (A). However, in the nerves treated with the HA-CMC solution, a minimal inflammatory cell infiltration and a mostly parallel appearance of axons were demonstrated at the repair site (C). The thickness of the blue-stained collagen depositions surrounding the nerve in the HA-CMC solution group was less than that in the saline group (B, D).

Fig. 9
Photomicrographs showing the longitudinal sections of the sciatic nerves following cut and repair with saline (A, B) and with the hyaluronic acid-carboxymethylcellulose (HA-CMC) solution (C, D) at 12 weeks after surgery. The sections were stained with H&E (A, C) and with Masson's trichrome (B, D). Original magnification was × 40. In the nerves treated with saline, a mild inflammatory cell infiltration and a mostly parallel appearance of axons were observed at the repair site (A). However, in the nerves treated with the HA-CMC solution, a minimal inflammatory cell infiltration and a normal looking appearance of axons were demonstrated at the repair site (C). Compared to the HA-CMC solution group, the marked deposition of collagen surrounding the nerve was demonstrated in the saline group (B, D).
Fig. 10
Results of the scar tissue formation index. The scar tissue formation index was significantly decreased in the experimental group at 12 weeks as compared with that in the control group (*p < 0.05).

References
1. Rydevik B, Lundborg G, Nordborg C. Intraneural tissue reactions induced by internal neurolysis: an experimental study on the blood-nerve barrier, connective tissues and nerve fibres of rabbit tibial nerve. Scand J Plast Reconstr Surg. 1976. 10(1):3–8.
2. Wilgis EF, Murphy R. The significance of longitudinal excursion in peripheral nerves. Hand Clin. 1986. 2(4):761–766.

3. Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986. 119(2):219–234.

4. Wang KK, Nemeth IR, Seckel BR, et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998. 18(4):270–275.

5. Burns JW, Skinner K, Colt J, et al. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995. 59(6):644–652.

6. Schimizzi AL, Massie JB, Murphy M, et al. High-molecularweight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J. 2006. 6(5):550–556.

7. Ikeda K, Yamauchi D, Osamura N, Hagiwara N, Tomita K. Hyaluronic acid prevents peripheral nerve adhesion. Br J Plast Surg. 2003. 56(4):342–347.

8. Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003. 23(6):575–581.

9. Isik S, Ozturk S, Gurses S, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg. 1999. 52(5):373–379.

10. Yamamoto M, Endo N, Ito M, et al. Novel polysaccharide-derived hydrogel prevents perineural adhesions in a rat model of sciatic nerve adhesion. J Orthop Res. 2010. 28(3):284–288.

11. Rodgers KE, Robertson JT, Espinoza T, et al. Reduction of epidural fibrosis in lumbar surgery with Oxiplex adhesion barriers of carboxymethylcellulose and polyethylene oxide. Spine J. 2003. 3(4):277–283.

12. Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronatebased bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996. 183(4):297–306.
13. Adanali G, Verdi M, Tuncel A, Erdogan B, Kargi E. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg. 2003. 19(1):29–36.

14. Magill CK, Tuffaha SH, Yee A, et al. The short- and long-term effects of Seprafilm on peripheral nerves: a histological and functional study. J Reconstr Microsurg. 2009. 25(6):345–354.

15. Lew DH, Yoon JH, Hong JW, Tark KC. Efficacy of antiadhesion barrier solution on periimplant capsule formation in a white rat model. Ann Plast Surg. 2010. 65(2):254–258.

16. Petersen J, Russell L, Andrus K, MacKinnon M, Silver J, Kliot M. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery. 1996. 38(5):976–983.

17. Brown RE, Erdmann D, Lyons SF, Suchy H. The use of cultured Schwann cells in nerve repair in a rabbit hind-limb model. J Reconstr Microsurg. 1996. 12(3):149–152.

18. Jones NF, Shaw WW, Katz RG, Angeles L. Circumferential wrapping of a flap around a scarred peripheral nerve for salvage of end-stage traction neuritis. J Hand Surg Am. 1997. 22(3):527–535.

19. Dumanian GA, McClinton MA, Brushart TM. The effects of free fat grafts on the stiffness of the rat sciatic nerve and perineural scar. J Hand Surg Am. 1999. 24(1):30–36.

20. Xu J, Varitimidis SE, Fisher KJ, Tomaino MM, Sotereanos DG. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J Hand Surg Am. 2000. 25(1):93–103.

21. Finsterbush A, Porat S, Rousso M, Ashur H. Prevention of peripheral nerve entrapment following extensive soft tissue injury, using silicone cuffing: an experimental study. Clin Orthop Relat Res. 1982. (162):276–281.
22. Nachemson AK, Lundborg G, Myrhage R, Rank F. Nerve regeneration and pharmacological suppression of the scar reaction at the suture site: An experimental study on the effect of estrogen-progesterone, methylprednisolone-acetate and cis-hydroxyproline in rat sciatic nerve. Scand J Plast Reconstr Surg. 1985. 19(3):255–260.

23. Atkins S, Smith KG, Loescher AR, Boissonade FM, Ferguson MW, Robinson PP. The effect of antibodies to TGF-beta1 and TGF-beta2 at a site of sciatic nerve repair. J Peripher Nerv Syst. 2006. 11(4):286–293.

24. Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007. 68(6):615–622.

25. Albayrak BS, Ismailoglu O, Ilbay K, et al. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: outcome based on gross postsurgical, histopathological, and ultrastructural findings. J Neurosurg Spine. 2010. 12(3):327–333.

26. Hiro D, Ito A, Matsuta K, Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986. 140(2):715–722.

27. Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987. 30(7):769–778.

28. Dam-Hieu P, Lacroix C, Said G, Devanz P, Liu S, Tadie M. Reduction of postoperative perineural adhesions by Hyaloglide gel: an experimental study in the rat sciatic nerve. Neurosurgery. 2005. 56:2 Suppl. 425–433.

29. Gago LA, Saed GM, Chauhan S, Elhammady EF, Diamond MP. Seprafilm (modified hyaluronic acid and carboxymethylcellulose) acts as a physical barrier. Fertil Steril. 2003. 80(3):612–616.

30. Klingler PJ, Floch NR, Seelig MH, Branton SA, Wolfe JT, Metzger PP. Seprafilm-induced peritoneal inflammation: a previously unknown complication: report of a case. Dis Colon Rectum. 1999. 42(12):1639–1643.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download