Abstract
Background
Porous β-calcium pyrophosphate (β-CPP) was developed to improve the fusion success of posterolateral lumbar fusion (PLF). The possibility of accomplishing PLF using a mixture of porous β-CPP and iliac bone was studied. This paper reports the radiologic results of PLF using the β-CPP plus autograft for lumbar degenerative disease as a bone graft extender.
Methods
A prospective, case-matched, radiographic study evaluating the results of short segment lumbar fusion using a β-CPP plus autograft was performed to compare the efficacy of β-CPP plus autograft with that of an autograft alone for short segment lumbar fusion. Thirty one consecutive patients (46 levels) underwent posterolateral fusion with pedicle screw fixation and additional posterior lumbar interbody fusion. In all patients, 3 mL of β-CPP plus 3 mL of autogenous bone graft was placed randomly in one side of a posterolateral gutter, and 6 mL of autogenous iliac bone graft was placed on the other. The fusion rates, volumes of fusion masses, and bone absorption percentage were evaluated postoperatively using simple radiographs and 3 dimensional computed tomography (3D-CT) scans.
Results
The control sides treated with an autograft showed significantly better Lenke scores than the study sides treated with β-CPP at 3 and 6 months postoperatively, but there was no difference between the two sides at 12 months. The fusion rates (confirmed by 3D-CT) were 87.0% in the β-CPP group and 89.1% in the autograft group, which were not significantly different. The fusion mass volumes and bone absorption percentage at 12 months postoperatively were 2.49 mL (58.4%) and 1.89 mL (69.5%) for the β-CPP and autograft groups, respectively, and mean fusion mass volume was significantly higher in the β-CPP group.
Posterolateral lumbar arthrodesis is used to treat many spinal conditions, such as scoliosis, stenosis, spondylolisthesis and degenerative disc disease. Its two primary shortcomings are the morbidity associated with the harvesting of an autogenous iliac bone graft,1-4) and the risk of pseudarthrosis.4) Calcium phosphate ceramics, such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP), are well known bone graft extenders that exhibit bioactivity, and form chemical bonds directly with natural bone. On the other hand, these two types of calcium phosphate ceramics have drawbacks. HA has excellent osteoconductivity and biocompatibility but the resorption rate is so low that it remains in the living body for a long time. β-TCP is biodegradable and might not provide the temporary persistence required as a scaffold for the formation of bone.5) A low fusion rate of β-TCP compared to that of HA was observed in the rabbit posterolateral fusion model.6) Accordingly, if ceramics with the same degree of osteoconductivity as HA, and lower biodegradability than β-TCP but better than that of HA can be developed, they might be a more ideal bone graft extender.
Studies have shown that β-calcium pyrophosphate (β-CPP) provides an alternative graft material that is bioactive, non-toxic7) and is incorporated more completely and resorbed more rapidly than porous HA in a canine model.5) Moreover, β-CPP has an excellent fusion rate compared to several other porous calcium phosphate ceramics in a rabbit intertransverse process fusion model,6) and that it also has a positive effect as a coating material during the initial stages after titanium screw fixation in a canine model.8) On the other hand, few reports are available on the merits of β-CPP used as a bone graft extender. This prospective in vivo study compared the use of a porous synthetic β-CPP (Bongros®-CP) as a novel bone graft extender in a posterolateral spine fusion model with a 100% autograft.
A previous report on the use of HA described the methods used in this study in detail.9) Between May 2004 and May 2005, 33 consecutive patients (48 levels) with spinal stenosis, or grade I or grade II spondylolisthesis, who underwent decompression and one- or two-level pedicle screw instrumented spinal fusion, were recruited prospectively after obtaining approval from the institutional review board and the Korean Food and Dug Administration. Informed consent was obtained from all participants. All patients were treated by the same operation team and underwent combined interbody fusion with the placement of 2 titanium alloy cages using local morselized bone for one level and additional posterolateral fusion using β-CPP with an iliac bone graft on one side and iliac bone graft on the other side. The inclusion criteria were lumbar posterior interbody fusion and posterolateral fusion with pedicle screw fixation. The exclusion criteria were an infection, malignancy, pregnancy, abnormal laboratory findings or metabolic bone disease, a liver function abnormality, or aged over 75 or under 18.9) The mean age of the patients was 63.3 (range, 46 to 73 years), and there were 25 women and 8 men. All patients were followed up for at least 12 months.
Bongros®-CP (Bioalpha, Seongnam, Korea) is composed of highly pure, synthetic calcium pyrophosphate (Ca2P2O7), and has a trabecular structure that resembles the 3-dimensional interconnected pore structure of human cancellous bone (pore size, 300 µm; porosity, 80%; particle size, 3.0 to 6.0 mm) (Fig. 1).
A standard posterior lumbar interbody fusion technique with laminectomies, medial facetectomies, discectomies, and transpedicular screw/rod instrumentation was performed on all patients. Autografts, which were harvested locally during decompression, were morselized and packed into the disc spaces with titanium alloy cages. Autogenous iliac crest bone was also harvested and morselized. Decortication of the transverse processes and lateral facet surfaces were performed before β-CPP with an autograft mixture or autograft bone implantations.10-12)
The test material (3 mL of β-CPP and 3 mL of autogenous iliac bone) was inserted in one side and the control material (6 mL of autogenous iliac bone) was inserted into the other side in each patient in a random manner. Therefore, the patients served as their own controls.13) The postoperative management protocols were the same for all patients including perioperatice pain control.9)
Radiographic evaluation of fusion using plane radiographs with standing lumbosacral anteroposterior, standing lateral, standing lateral flexion, and standing lateral extension images were made 3, 6, and 12 months postoperatively. Three dimensional thin cut (1 mm) CT images were obtained at 12 months postoperatively and reconstructed axially, sagittally and coronally. All imaging studies were evaluated for evidence of fusion by three independent orthopedic surgeons in a blind manner. The CT assessments of fusion were described in our previous report9) including continuous trabecular bone between transverse processes, cortication at the peripheral edges of fusion masses, and of the absence of identifiable radiographic clefts (Fig. 2).14) The instrumented spinal level were considered when three observers found no radiographic evidence of nonunion. Fusion of the sides was defined as bridging at all levels. Fusions were classified using Lenke's classification15) at 3, 6, and 12 months postoperatively. At 12 months, the volume of the fusion masses on either side of the vertebrae were assessed by 3 dimensional computed tomography (3D-CT) scans and compared using Rapidia (version 2.8, Infinitt, Seoul, Korea). To measure the volume of the fusion mass, sequential CT scans with a 1 mm collimation and 1 mm scan spacing were obtained 12 months after surgery.9) To measure the volume of the fusion mass, two independent spine surgeons measured the area of the fusion mass using the manual cursor technique and the volume of the fusion mass was summated at each cross-sectional volume which was estimated using the cross-sectional area multiplied by the slice thickness (1 mm).9,11) The absorption percentage of the autograft β-CPP were also calculated by the same method of previous report.9)
The vital signs, subjective symptoms and laboratory results were evaluated preoperatively and 3, 6, and 12 months after surgery. A paired-sample t-test was used to identify significant radiological differences between the two treatments. Statistical significance was accepted at the p < 0.05 level, and the analysis was performed throughout using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
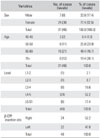
Overall, out of 33 patients (48 segments), a total of 31 (46 segments) were available for a 3D-CT evaluation at the 12 month follow-up. Table 1 lists the final patient demographics. The mean Lenke score on the 3-month follow-up radiograph in the autograft group (1.35) was significantly lower than that of the β-CPP + autograft mixture group (2.15; p < 0.01), as were Lenke scores at the 6-month follow-up (1.13 vs. 1.47, respectively; p < 0.01), but not at 12 months (1.13 vs. 1.22, respectively; p > 0.05) (Table 2). On the other hand, the Lenke scores, which were confirmed by the 3D-CT scans at 12 months, were 1.2 and 1.22 for autograft and β-CPP + autograft group, respectively, which was not significantly different (p > 0.05).
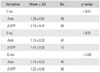
The number of levels showing complete fusion in the autograft and β-CPP + autograft group at 12 months was 41 (89.1%) and 40 (87.0%), respectively, which was not significantly different. The mean fusion mass volumes and mean fusion mass absorption percentage were significantly different in the two groups at 12 months postoperatively according to 3D-CT. In particular, the mean volume in the autograft group (1.89 mL) was significantly lower (p < 0.01) than that in the β-CPP + autograft group (2.49 mL), and the mean absorption percentage in the autograft group (69.5%) was also significantly higher (p < 0.01) than that in the β-CPP + autograft group (58.4%) (Table 3). The mean absorption percentage of β-CPP was 48.5% and its mean relative absorption percentage versus the autograft was 69.8%.
No definite postoperative complications, e.g., infection, abnormal vital signs or abnormal lab findings (including calcium and phosphorous levels), were encountered in either group.
Calcium phosphate ceramics, such as Ca10(PO4)6(OH)2(HA), β-Ca3(PO4)2(β-TCP), and β-Ca2P2O7, are bioactive, or more specifically have the ability to form chemical bonds directly with natural bone.16-19) Transmission electron microscopy of the interfaces of bone and Ca2P2O7 ceramics has shown that β-CPP is biocompatible, like β-TCP and HA.15) HA and other complex calcium phosphate salts are the end products of the biological mineralization process, and β-CPP is formed during this process,20) and has a biological response in terms of new bone development, which is similar to that of HA.17) Some researchers view β-CPP as a new biodegradable ceramic material21-23) because it is more biocompatible than hydroxyapatite.22) The Ca/P ratio of β-CPP is 1, which is far lower than that of β-TCP, and is potentially more biodegradable than β-TCP. On the other hand, β-TCP is more biodegradable than β-CPP and β-CPP is more biodegradable than HA. Therefore, β-CPP was selected as a new bone graft extender.
In the present study, clinical assessments (e.g., Visual Analogue Scale pain score, Short Form-36, and Oswestry Disability Index) were not included because the test material and control material was inserted on opposite sides in the same patients.
The most important parameter in terms of an evaluation of the effectiveness of newly manufactured bone graft extenders is the fusion rate. Therefore, precise assessments of the fusion rates are critical, and the use of fine-cut CT scans with sagittal and coronal reconstructions might increase the accuracy of fusion assessments.24) Therefore, the use of 3D-CT to evaluate the presence of a successful arthrodesis is a major strength of the present study because thin-section CT scans allow bony discontinuities to be discerned more easily.14)
One problem that should be considered is whether the posterolateral fusion rates were increased slightly by the additional posterior lumbar interbody fusion with cage insertion. The use of supplementary instrumentation is controversial, as the fusion rates may be improved.25-28) In the present study, any additional stability would have affected the fusion rates on both sides equally. Hence, cage insertion would not be expected to alter the relative fusion rates of the two groups. The other problem is whether additional posterior lumbar interbody fusion was needed in this study. Because β-CPP has not been used previously in humans, the posterolateral spinal fusion rates could not be estimated. Consequently, this study was designed intentionally to minimize the risk of nonunion by introducing additional posterior lumbar interbody fusion and to evaluate the effectiveness of β-CPP by adding a minimal amount of autograft. Indeed, the fusion rates in the intertransverse process area in another study varied widely from 60% to 98%.29) On the other hand, the fusion rate of the autograft in the present study was only 89.1% despite the additional cage insertion, which is probably due to the insertion of a relatively small amount (6 mL) of autograft. Moreover, the additional interbody fusion by the cages imparted strong stability to both posterolateral fusion masses to the same degree. Although this instrumentation cannot eliminate the flaws in this study design, pedicle screw and rod fixation with additional interbody fusion with cages might minimize the effects of fusion on one side on fusion on the other side.9)
The most important result of the present study was that the fusion rates, as confirmed by the 3D-CT scans, were similar in the two groups, which indicates that β-CPP provides adequate fusion. This signifies the possibility of a mixture of β-CPP and autograft being used as bone graft extender. The absorption percentage of β-CPP (48.5%) was significantly lower than that of the autograft (69.5%) but higher than that of HA (43.5%) based on our institute's data.9) Moreover, the relative absorption percentage of β-CPP versus the autograft (69.8%) was also higher than that of HA versus autograft (55.6%).7) This may be the advantage of β-CPP over relatively slowly absorbed bone graft extenders, such as HA or apatite-wollastonite glass-ceramics. The advantages of biodegradable materials are evident in that no foreign body remains in the long term. Moreover, after the porous ceramic material has degraded, the remodeled bone is stronger than a ceramic/newly formed bone combination.30) Complete biological and physiologic bone healing can be achieved by implanting biodegradable bioceramics. Although β-TCP, calcium carbonate (CaCO3), and calcium sulfate (CaSO4) are biodegradable, none of these are effective bone graft extenders for spinal intertransverse process fusion.
In view of the above, rapid absorption must be matched by excellent osseointegration, even in difficult fusion beds, such as those that have been infected or radiated, and be located near scar tissue, or extraosseous fusion beds, such as the intertransverse process area. To achieve successful fusion in such difficult environments, the osteoconductivity of the implanted material and the maintenance of porous structures until bone ingrowth has occurred from the fusion bed into the graft pores are critical. Moreover, β-CPP has excellent osteoconductivity and the property of maintaining a porous structure, which are the reasons why β-CPP can be used during posterolateral fusion of the spine.
In conclusion, the described β-CPP/autograft mix achieved successful posterolateral spinal fusion in the presence of instrumentation, and it has absorption properties. There were no signs of toxicity or abnormal laboratory findings in any patient in this study. Therefore, β-CPP can be used as a novel bone graft extender for intertransverse process fusion with short-segment instrumentation.
Figures and Tables
 | Fig. 1Bongros-CP. (A) Macroscopic appearance of Bongros-CP. (B) Scanning electron microscopy image of Bongros-CP. |
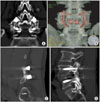 | Fig. 2Three dimensional computed tomography (3D-CT) scan 12 months after an L4-L5 instrumented lumbar fusion utilizing a β-tricalcium phosphate (β-TCP) + autograft (calcium pyrophosphate, CPP) and autograft (Auto). Note the continuity of the β-CPP and bone fragments over the transverse processes. (A) Coronal reconstruction of β-CPP. (B) Coronal reconstruction of autograft. (A) and (B), coronal reconstruction of a CT scan demonstrates the incorporation of β-CPP through the matrix. (C) Sagittal reconstruction of β-CPP. (D) Sagittal reconstruction of the autograft. (C) and (D), Sagittal reconstruction of a CT scan demonstrates that the continuous bony mass is present bilaterally with no evidence of lucent lines. |
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid for Common core technology development program from the Korean Ministry of Knowledge Economy (No. A00-A01-3302-02-1-3).
References
1. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996. (329):300–309.

2. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res. 1997. (339):76–81.

3. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine (Phila Pa 1976). 1995. 20(9):1055–1060.
4. Boden SD, Grob D, Damien C. Ne-Osteo bone growth factor for posterolateral lumbar spine fusion: results from a nonhuman primate study and a prospective human clinical pilot study. Spine (Phila Pa 1976). 2004. 29(5):504–514.

5. Lee JH, Lee DH, Ryu HS, Chang BS, Hong KS, Lee CK. Porous beta-calcium pyrophosphate as a bone graft substitute in a canine bone defect model. Key Eng Mater. 2003. 240-2:399–402.

6. Lee DH, Ryu HS, Lee SL, et al. Comparison of oteosyntheses in various types of porous calcium phosphate compounds: an experimental study by posterolateral fusion of rabbits' lumbar vertebrae. J Korean Soc Spine Surg. 2001. 8(4):455–467.

7. Lee JH, Chang BS, Ryu HS, Lee CK. A 90-day subchronic toxicity study of beta-calcium pyrophosphate in rat. Drug Chem Toxicol. 2009. 32(3):277–282.

8. Lee JH, Ryu HS, Lee DS, Hong KS, Chang BS, Lee CK. Biomechanical and histomorphometric study on the bone-screw interface of bioactive ceramic-coated titanium screws. Biomaterials. 2005. 26(16):3249–3257.

9. Lee JH, Hwang CJ, Song BW, Koo KH, Chang BS, Lee CK. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009. 90(3):804–810.

10. Fujibayashi S, Shikata J, Tanaka C, Matsushita M, Nakamura T. Lumbar posterolateral fusion with biphasic calcium phosphate ceramic. J Spinal Disord. 2001. 14(3):214–221.

11. Gunzburg R, Szpalski M. Use of a novel beta-tricalcium phosphate-based bone void filler as a graft extender in spinal fusion surgeries. Orthopedics. 2002. 25:5 Suppl. s591–s595.
12. Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991. 73(6):802–808.

13. Alexander DI, Manson NA, Mitchell MJ. Efficacy of calcium sulfate plus decompression bone in lumbar and lumbosacral spinal fusion: preliminary results in 40 patients. Can J Surg. 2001. 44(4):262–266.
14. Singh K, Smucker JD, Gill S, Boden SD. Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech. 2006. 19(6):416–423.

15. Lenke LG, Bridwell KH, Bullis D, Betz RR, Baldus C, Schoenecker PL. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992. 5(4):433–442.

17. Kitsugi T, Yamamuro T, Nakamura T, Kotani S, Kokubo T, Takeuchi H. Four calcium phosphate ceramics as bone substitutes for non-weight-bearing. Biomaterials. 1993. 14(3):216–224.

18. Kitsugi T, Yamamuro T, Nakamura T, Oka M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials. 1995. 16(14):1101–1107.

19. Kasuga T, Sawada M, Nogami M, Abe Y. Bioactive ceramics prepared by sintering and crystallization of calcium phosphate invert glasses. Biomaterials. 1999. 20(15):1415–1420.

20. Ducheyne P. Bioceramics: material characteristics versus in vivo behavior. J Biomed Mater Res. 1987. 21:A2 Suppl. 219–236.
21. Lin FH, Lin CC, Lu CM, Liu HC, Sun JS, Wang CY. Mechanical properties and histological evaluation of sintered beta-Ca2P2O7 with Na4P2O7.10H2O addition. Biomaterials. 1995. 16(10):793–802.

22. Sun JS, Tsuang YH, Liao CJ, Liu HC, Hang YS, Lin FH. The effects of calcium phosphate particles on the growth of osteoblasts. J Biomed Mater Res. 1997. 37(3):324–334.

23. Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res. 1983. 17(5):769–784.

24. Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976). 2006. 31(22):2534–2539.

25. Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993. 6(6):461–472.

26. Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997. 22(24):2807–2812.
27. Nork SE, Hu SS, Workman KL, Glazer PA, Bradford DS. Patient outcomes after decompression and instrumented posterior spinal fusion for degenerative spondylolisthesis. Spine (Phila Pa 1976). 1999. 24(6):561–569.

28. Rechtine GR, Sutterlin CE, Wood GW, Boyd RJ, Mansfield FL. The efficacy of pedicle screw/plate fixation on lumbar/lumbosacral autogenous bone graft fusion in adult patients with degenerative spondylolisthesis. J Spinal Disord. 1996. 9(5):382–391.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download