Abstract
Background
We analyzed the radiological and clinical results of our study subjects according to the management algorithm of the Vancouver classification system for the treatment of periprosthetic femoral fractures in hip arthroplasty.
Methods
We retrospectively reviewed 18 hips with postoperative periprosthetic femoral fractures. The average follow-up was 49 months. The fracture type was determined based on the Vancouver classification system. The management algorithm of the Vancouver classification system was generally applied, but it was modified in some cases according to the surgeon's decision. At the final follow-up, we assessed the radiological results using Beals and Tower's criteria. The functional results were also evaluated by calculating the Harris hip scores.
Results
Seventeen of 18 cases (94.4%) achieved primary union at an average of 25.5 weeks. The mean Harris hip score was 92. There was 1 case of nonunion, which was a type C fracture after cemented total hip arthroplasty, and this required a strut allograft. Subsidence was noted in 1 case, but the fracture was united despite the subsidence. There was no other complication.
The incidence of periprosthetic femoral fractures after hip arthroplasty is increasing as a result of the increased performance of total hip arthroplasty (THA), the aging population and complications such as osteolysis and aseptic loosening.1) The treatment of periprosthetic femoral fractures requires particular care because secure fixation is difficult in this situation as compared to ordinary fractures and the instability of a femoral stem may affect the durability of the artificial joint. Therefore, much effort has been expended to properly classify and treat periprosthetic femoral fractures. A classification system based on the fracture pattern was first proposed.2) After that, additional criteria were suggested such as the timing of the fracture, the location of fracture and the prosthetic stability.3) Nowadays, the Vancouver classification system4) (Table 1) is universally applicable, and this system unites the advantages of the existing classification systems with considering the bone quality. This system is reported to be reliable and valid.5) Yet practically, it is not easy to strictly apply the rules in some cases because there is no objective standard to assess prosthetic stability or bone quality. Sometimes the choice of an internal fixation device to employ for a unique fracture pattern may come into question. In this study we compared the management algorithm of the Vancouver classification system with what we did to treat periprosthetic femoral fractures that occurred after hip arthroplasty, and we analyzed the radiological and clinical results. We also review the relevant literature.
We retrospectively reviewed a consecutive series of 41 periprosthetic femoral fractures that occurred after hip arthroplasty between March 1993 and October 2009. Thirteen intraoperative fractures were excluded from this study. Three cases were lost to follow-up. Two cases of the repair of nonunion that were transferred from other institutes and 5 cases with follow-up periods less than 6 months were excluded. Thus, the final study group consisted of 18 cases (18 hips). There were 10 men and 8 women. The average patient age was 58.8 years (range, 38 to 87 years). The status of the arthroplasties was THA in 14 and bipolar hemiarthroplasty in 4. There were 17 primary arthroplasties and 1 revision arthroplasty. The femoral components in place at the time of surgery included a cemented femoral component in 7 hips and a cementless femoral component in 11 hips. The fractures were classified according to the Vancouver system. The operations were performed by a single surgeon in all cases. The management algorithm of the Vancouver classification system was generally applied, but it was modified in some cases according to the surgeon's decision and the operative field. A comparison of the management algorithm of the Vancouver classification system with what we did is summarized in Table 2. We will subsequently go into details in the Discussion Section. The causes of fractures and the time to fractures after hip arthroplasties were investigated via the medical records. Radiological evaluations were conducted using Beals and Towers' criteria (Table 3).6) At the final follow-up, clinical evaluations were performed using the Harris hip score, which was regarded as satisfactory if the score was more than 80.7)
Ten hips were classified as Vancouver type B1, 3 were type B2, 1 was type B3 and 4 were type C. There was no type A fracture. The causes of fractures were a fall in 15 and a traumatic accident in 3. The average time to fractures after hip arthroplasties was 49 months (range, 0.25 to 135 months).
In this study, the type B1 fractures were treated with cerclage wiring alone in 3 hips, and they were fixed with a plate or cortical strut allograft in 7 hips. All the type B2 fractures were treated with revision to a long stem. Among them, 2 cases were each reinforced with a plate and a cortical strut allograft (Fig. 1). One type B3 fracture was treated with revision and augmentation with a plate instead of an allograft. All the type C fractures were treated with the fixation of fractures (Table 2).
Complete fracture union occurred in 17 of the 18 cases (94.4%) at an average of 25.5 weeks (range, 12 to 68 weeks). The average time to union according to the fracture classification was 23.2 weeks for the type B1 fractures, 28.7 weeks for the type B2 fractures, 26.9 weeks for a type B3 fracture and 15.7 weeks for the type C fractures. The time to union was different even in the same type of fracture according to the treatment options. In type B1 fractures, cerclage wiring alone achieved union at an average of 26.4 weeks, while fixation with a plate or cortical strut allograft achieved union at an average of 15.7 weeks. For the type B2 fractures, revision to a long stem alone achieved union at 52.1 weeks, while revision reinforced with a plate or a cortical strut allograft achieved union at an average of 17 weeks. The type B3 fracture treated with revision and augmentation with a plate achieved union at 26.9 weeks. For the type C fractures, fixation with a plate achieved union at an average of 14.7 weeks, while fixation with a cortical strut allograft achieved union at 17.8 weeks. One case of nonunion occurred in a type C fracture treated with plate fixation in a cemented THA (Fig. 2). The fracture did not unite and it showed progressive varus deformity even at 10 months follow-up. This hip was managed with plate removal and fixation with a cortical strut allograft. The correlation of nonunion between the cemented and cementless stem was not significant statistically (p = 0.389, chi-square test). One case showed subsidence despite of a united fracture, but the patient refused further surgery and the patient remains under close observation. The radiological results using Beals and Towers' criteria were excellent in 16 hips, good in 1 and poor in 1. The overall clinical results were satisfactory and the average Harris hip score was 92 (range, 84 to 98). No other complications were identified.
The guideline for the treatment of type B1 fracture according to the Vancouver classification system is open reduction and internal fixation with various implants such as cerclage wire, a plate or a cortical strut allograft. Giannoudis et al.8) recommended cerclage wiring should be abandoned and only cables should be used as supplements of fixation plates or onlay allografts. In this study, fracture union certainly occurred earlier in the group treated with a plate or a cortical strut allograft rather than cerclage wiring alone. Nevertheless, cerclage wiring was able to achieve union in all of the fractures it was applied to. Therefore, the choice of implant does not seem to be closely associated with nonunion if stable fixation of the fracture can be performed for type B1 fracture. Care must be taken to preserve the periosteal blood supply so as not to cause nonunion. To circumvent this complication, Duncan and Masri9) preferred bone grafting during open reduction and internal fixation, while Ricci et al.10) recently reported favorable result using indirect reduction and plate fixation without bone grafting. The latter currently seems to be a good option for the treatment of type B1 fracture from now on.
The stability of the femoral stem should be kept in mind when treating periprosthetic femoral fractures in hip arthroplasty. Lindahl et al.11) proposed that the prosthesis should be considered as loose until proven otherwise because it is probable that many fractures classified as type B1 are in reality type B2 fractures with a loose stem, and the loose stem is not recognized. Lindahl et al.12) recommended exploration of the joint to test the stability of the implant in which the stability of the stem is uncertain. In this study, we conducted preoperative computed tomography scans and intraoperative checkup of the stability of the stem so as not to mistake type B2 fractures for type B1 fractures. There is no basis for objectively validating the stability of a femoral stem despite that this may cause a major change of the course in the management algorithm. This is thought to be a drawback of the Vancouver classification system. So, we strongly feel it is necessary to develop a definite concept to assess the stability of a femoral stem.
A guideline for the treatment of type B2 fracture according to the Vancouver classification system is revision to a long stem. In this study, 2 of the 3 type B2 fractures were reinforced with additional internal fixation, and in these 2 fractures fracture union occurred earlier than that with performing revision with a long stem alone. One case was a long spiral fracture from the middle portion of the femoral stem to the distal femur, in which a plate was reinforced because the long stem for revision could not adequately fix the distal fracture fragment. The other case was a spiral fracture from the proximal femur through the whole length of the femoral stem, in which a cortical strut allograft was used for reinforcement because the proximal femoral metaphyseal fixation of the long stem was not firm. Macdonald et al.13) reported favorable results using a similar method.
A guideline for the treatment of type B3 fracture according to the Vancouver classification system is revision and augmentation, for which various surgical options are available such as a complex reconstruction of the deficient proximal femur with secure distal fixation, segmental substitution of the proximal femur with a megaprosthesis or a allograft/stem composite, and distally fixed replacement with a modular stem.14) Nevertheless, 1 type B3 fracture was united by revision and augmentation with a plate and massive allogenic chip bone grafting in this study. Although this was an experience of just one case, we carefully suggest that that some type B3 fractures can be treated without a cortical strut allograft and with preserving the femur as much as possible, under the exact evaluation of the remaining femoral bone stock.
For the type C fractures, 1 fracture, which was between 2 fractures that were treated with plates, achieved union at 14.7 weeks; 1 other type C fracture was treated with a cortical strut allograft at 17.8 weeks. Early fracture union occurred in the case treated with a plate. We assumed two reasons for the one ununited type C fracture. First, an insufficient plate length to fix the distal fracture fragment may have shortened the working length. Under these circumstances, an anterior strut graft may produce a strong construct.15) Haddad et al.16) suggested that cortical strut grafts should be routinely used to augment fixation and healing of periprosthetic femoral fractures. Second, soft tissue stripping during open reduction may have injured even the periosteal blood supply under the circumstances that endosteal blood supply may have been disturbed because of bone cement filled in the medullary cavity. The interrelationship between bone cement and nonunion is uncertain. However, we consider that soft tissue should be handled more carefully during open reduction of cemented periprosthetic femoral fractures. We treated the nonunion with a cortical strut allograft. Barden et al.17) reported good outcomes in a similar way.
Early identification and appropriate intervention are critical to prevent periprosthetic femoral fractures because of the difficult reconstructive challenges. In many cases, fractures happen because of the previously unrecognized osteolysis and weakening of the bone as a result of loosening of the prosthesis stem. Thus, the key to prevention is routine follow-up with radiographic studies.18,19) Wedemeyer et al.20) reported that a radiograph taken prior to trauma showed an endosteal reaction at the level at which the fracture later occurred, which might have been an indication that the stem of the prosthesis was already broken. Thomsen et al.21) recommended cemented stems in the patients with poor bone quality and for whom a cementless hip stem is at a higher risk of periprosthetic fractures. Foster et al.22) suggested that cemented hemiarthroplasty is preferable, and especially in elderly frail patients, to reinforce an osteoporotic proximal femur.
The weakness of this study includes the lack of each fracture type. That notwithstanding, this study shows that the management algorithm of the Vancouver classification system can be modified in consideration of the existing state of fractures. To sum up, early fracture union occurred in the hips treated with fixation with a plate or cortical strut allograft rather than with cerclage wiring alone in type B1 fractures; revision and augmentation with a plate or a cortical strut allograft rather than revision to a long stem alone also achieved early fracture union for treating type B2 fractures. In type B3 fracture, revision and augmentation with a plate instead of using an allograft can achieve union. Custom-tailored treatment may be applicable according to the general medical condition of a patient, the stability of the femoral stem, the configuration of fractures and the socioeconomic status, and physicians should not blindly follow the routine management algorithm.
Figures and Tables
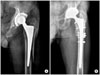 | Fig. 1(A) A Vancouver type B2 periprosthetic femoral fracture. (B) A radiograph showing union at 3 months follow-up after revision and augmentation with an allograft. |
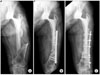 | Fig. 2(A) A Vancouver type C periprosthetic femoral fracture. (B) A radiograph showing nonunion with varus deformity at 10 months follow-up after open reduction and internal fixation with a plate. (C) Fracture union was identified at 4 months after revision with a cortical strut allograft. |
Table 1
Vancouver Classification System of Postoperative Periprosthetic Femoral Fractures

Modified from Masri et al.4) with permission.
Table 3
Beals and Towers' Criteria for Radiological Evaluation

Modified from Beals and Tower6) with permission.
References
1. Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury. 2007. 38(6):651–654.

2. Khan MA, O'Driscoll M. Fractures of the femur during total hip replacement and their management. J Bone Joint Surg Br. 1977. 59(1):36–41.

3. Johansson JE, McBroom R, Barrington TW, Hunter GA. Fracture of the ipsilateral femur in patients wih total hip replacement. J Bone Joint Surg Am. 1981. 63(9):1435–1442.

4. Masri BA, Meek RM, Duncan CP. Periprosthetic fractures evaluation and treatment. Clin Orthop Relat Res. 2004. (420):80–95.

5. Brady OH, Garbuz DS, Masri BA, Duncan CP. The reliability and validity of the Vancouver classification of femoral fractures after hip replacement. J Arthroplasty. 2000. 15(1):59–62.

6. Beals RK, Tower SS. Periprosthetic fractures of the femur: an analysis of 93 fractures. Clin Orthop Relat Res. 1996. 327:238–246.
7. Choi IY, Cho SH, Kim YH. Treatment of Vancouver B2 and B3 periprosthetic femoral fractures. J Korean Hip Soc. 2008. 20(2):110–116.

8. Giannoudis PV, Kanakaris NK, Tsiridis E. Principles of internal fixation and selection of implants for periprosthetic femoral fractures. Injury. 2007. 38(6):669–687.

9. Duncan CP, Masri BA. Fractures of the femur after hip replacement. Instr Course Lect. 1995. 44:293–304.
10. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. J Bone Joint Surg Am. 2005. 87(10):2240–2245.

11. Lindahl H, Malchau H, Oden A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006. 88(1):26–30.

12. Lindahl H, Garellick G, Regner H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am. 2006. 88(6):1215–1222.

13. Macdonald SJ, Paprosky WG, Jablonsky WS, Magnus RG. Periprosthetic femoral fractures treated with a long-stem cementless component. J Arthroplasty. 2001. 16(3):379–383.

14. Richards CJ, Garbuz DS, Masri BA, Duncan CP. Vancouver type B3 periprosthetic fractures: evaluation and treatment. Instr Course Lect. 2009. 58:177–181.
15. Howell JR, Masri BA, Garbuz DS, Greidanus NV, Duncan CP. Cable plates and onlay allografts in periprosthetic femoral fractures after hip replacement: laboratory and clinical observations. Instr Course Lect. 2004. 53:99–110.
16. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002. 84(6):945–950.
17. Barden B, Ding Y, Fitzek JG, Loer F. Strut allografts for failed treatment of periprosthetic femoral fractures: good outcome in 13 patients. Acta Orthop Scand. 2003. 74(2):146–153.

18. Franklin J, Malchau H. Risk factors for periprosthetic femoral fracture. Injury. 2007. 38(6):655–660.

19. Katzer A, Ince A, Wodtke J, Loehr JF. Component exchange in treatment of periprosthetic femoral fractures. J Arthroplasty. 2006. 21(4):572–579.

20. Wedemeyer C, Russe K, von Knoch M, Saxler G. Endosteal reaction in the region surrounding the stem of a cement-free prosthesis: an early radiological sign of imminent fracture of the femoral shaft? Unfallchirurg. 2007. 110(1):75–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download