Abstract
Congenital pseudarthrosis of the tibia (CPT) is one of the most challenging problems in pediatric orthopaedics. The treatment goals are osteosynthesis, stabilization of the ankle mortise by fibular stabilization, and lower limb-length equalization. Each of these goals is difficult to accomplish but regardless of the surgical options, the basic biological considerations are the same: pseudarthrosis resection, biological bone bridging of the defect by stable fixation, and the correction of any angular deformity. The Ilizarov method is certainly valuable for the treatment of CPT because it can address not only pseudarthrosis but also all complex deformities associated with this condition. Leg-length discrepancy can be managed by proximal tibial lengthening using distraction osteogenesis combined with or without contralateral epiphysiodesis. However, treatment of CPT is fraught with complications due to the complex nature of the disease, and failure is common. Residual challenges, such as refracture, growth disturbance, and poor foot and ankle function with stiffness, are frequent and perplexing. Refracture is the most common and serious complication after primary healing and might result in the re-establishment of pseudarthrosis. Therefore, an effective, safe and practical treatment method that minimizes the residual challenges after healing and accomplishes the multiple goals of treatment is needed. This review describes a multi-targeted approach for tackling these challenges, which utilizes the Ilizarov technique in atrophic-type CPT.
Congenital pseudarthrosis of the tibia (CPT) is not a homogeneous entity but is composed of several disease types1,2) with different prognoses.3-6) The precise cause of CPT is unclear, even though it appears to be related to neurofibromatosis-1 (NF-1). The natural history of CPT varies and is unpredictable,1,2,7) and no surgical or medical option appears to be capable of altering its natural history or pathobiology. The treatment aims are to achieve bony union without axial or rotational malalignment, stabilize the ankle mortise for good foot and ankle function, and achieve lower limb-length equalization. In atrophic-type CPT, these goals are extremely difficult to accomplish. However, primary union appears to have been improved markedly by modern treatment methods, such as, intramedullary fixation,8-11) microvascular transfer of a fibular graft,12,13) and external fixation using the Ilizarov technique.4-6,14-18) Nevertheless, regardless of the surgical options, the basic biological considerations of surgery are the same; pseudarthrosis resection, biological bone bridging of the defect with stable fixation, and correction of any angular deformity. However, residual challenges are commonly encountered and are perplexing. Moreover, occasionally, the residual problems encountered after primary union may be severe enough to jeopardize function of the affected extremity, particularly in atrophic-type CPT. Therefore, to obtain a stable, functional extremity at the completion of treatment, it is essential to set realistic goals and to adhere to the treatment principles and technical details. This review discusses how to accomplish multiple targets using the Ilizarov technique while minimizing the residual challenges after primary healing.
The Ilizarov method was introduced in the western world during the late 1980s, and has been widely popularized and applied to the treatment of CPT, because it can address pseudarthrosis as well as all components of the complex deformities associated with this condition. Furthermore, in contrast to other treatment modalities, the Ilizarov technique is not precluded by previous surgery, and the Ilizarov device can be reapplied in the event of refracture.5,15) The technique is particularly beneficial when other methods have failed or in patients with angulation threatening refracture, shortening exceeding 5 cm, late symptomatic ankle or proximal tibial valgus, proximal fibular migration, or procurvatum producing a severe calcaneus-type gait.19) In addition, because the Ilizarov technique rarely burns bridges,20) all other conventional treatment modalities are still possible if this technique fails. If necessary, a combination of two or three techniques can be used sequentially or simultaneously in an effort to obtain union. For example, a free vascularized fibula graft can be combined using the Ilizarov technique, thereby offering the advantage of addressing both bone loss and leg length inequality.21) For these reasons, many surgeons agree that the Ilizarov method is a viable surgical alternative. However, it is not the ultimate solution for all types of CPT.
A variety of techniques based on several different frame configurations and strategies have been reported for the Ilizarov method.4-6,17,18,22,23) Although the primary aim of treatment is union of the pseudarthrosis site, the therapeutic considerations should include other associated problems, such as leg-length discrepancy, multilevel and multidirectional tibial deformity, foot deformity, associated fibular pseudarthrosis and subsequent ankle valgus.22,24,25) According to the literatures produced by retrospective case series, the overall union rate for the Ilizarov technique ranges from 60 to 100%.20) However, the union rate and function after an Ilizarov treatment can be confounded by a variety of factors, such as the severity of dysplasia, failed previous treatments, and co-existing deformities.
The basic principles of the Ilizarov treatment for atrophic-type CPT include the following: a) meticulous and complete resection of the sclerotic bone edges and surrounding fibrous hamartoma, and reopening of the medullary canals; b) the restoration and maintenance of axial alignment and joint orientation by correcting all angular deformities; c) maximization of the cross-sectional area of healing at the level of the pseudarthrosis; d) ankle stabilization to prevent deformities around the ankle, such as ankle valgus and procurvatum of the distal tibia; e) stable construction of the ring fixator coupled with transfixing wires as far from the sclerotic lesion as possible; f) protective bracing or intramedullary nailing to prevent refracture after primary bone healing; and g) length-gain by distraction osteogenesis, preferably to < 2-3 cm of shortening, such that the residual discrepancies can be managed by shoe lifting and contralateral epiphysiodesis.
Complications attributable to treatment and the disease have been recognized. The complications related primarily to the use of an external device include pin-track infection, loosening, breakage, refracture at the pin insertion site, neurovascular injury, axial deviation, osteopenia and joint stiffness. Residual limb length discrepancy and valgus deformity are commonly reported with an overall complication rate of 30 to 100%.4,16)
Refracture is the most serious complication that may result in the re-establishment of pseudarthrosis. Indeed, there is a CPT subgroup that continues to cause refracture due to severe dysplasia.19) With regard to the risk factors of refracture after initial union in CPT, a younger age at surgery, low cross-sectional area of the healed segment, tumor recurrence, persistent fibular pseudarthrosis, residual ankle valgus, removal of an intramedullary rod, and non-compliance with bracing have been suggested.22,26) Inan et al.27) reviewed the residual deformities in 16 patients treated for CPT. Ten had diaphyseal malalignment, of which half experienced refracture. In another series, in which an intramedullary rod was used, Fern et al.28) reported a 40% refracture rate after 4.5 years, and Joseph and Mathew29) reported a 25% refracture rate after 3 years. We also investigated the patterns of refracture and their risk factors in 23 patients with atrophic-type CPT. Twenty patients had refracture after initial union.26) The refracture-free rate of cumulative survival was 47% at five years and did not change thereafter. The risk of refracture was significantly higher when osteosynthesis was performed below the age of four years, when the tibial cross-sectional area was low, and when associated with persistent fibular pseudarthrosis.26)
Some prognostic factors for CPT have been reported but there is considerable controversy, e.g., whether or not there is a correlation exists between the age at surgery and the final outcome. Although some authors have claimed that surgery under 3 to 4 years of age is beneficial,18,30) many others16,31) have recommended waiting until the child is 5 to 6 years of age because they observed higher success rates in older children. Morrissy et al.32) concluded that a good result is unlikely in 6 year old patients that have failed to achieve union. The use of Ilizarov methods in children younger than 5 years has either failed to achieve union or resulted in almost immediate refracture.4,6,31) A much higher refracture risk was also observed in patients younger than 4 years of age after the Ilizarov treatment compared to those older than 4 years, even though there was no difference in union rates according to age.26) Based on this observation, we now prefer to perform definitive Ilizarov surgery on patients older than 3 years.
The importance of fibular stabilization is emphasized because ankle valgus deformity caused by deficient lateral fibular support due to a fracture and/or concomitant fibular pseudarthrosis, or distal tibial wedging is strongly related to refracture.2) Tudisco et al.3) reported the functional results of 30 CPT patients at the end of skeletal growth, and concluded that the prognosis of CPT is related to the radiologic findings; patients with a severe limb length discrepancy, poor ankle function, and fibular pseudarthrosis had the poorest functional results. Other authors also reported that persistent fibular pseudarthrosis is related to tibial union failure8,11) and progressive ankle valgus.6) Dobbs et al.9) also found in their long-term follow-up study on the use of an intramedullary rod to treat CPT that most ankle valgus deformities occurred in patients with concomitant fibular pseudarthrosis, even after the fibular lesion had been treated. In a previous study,26) based on a survival analysis approach, fibular stabilization by either tibiofibular synostosis or fibular osteosynthesis resulted in significantly better refracture-free survival than that experienced by those with a pseudarthrotic fibula due to neglect or failed synostosis. The hazard ratio of refracture was 3.997 (95% confidence interval [CI], 1.218 to 13.119) with fibular stability, but only 0.986 (95% CI, 0.977 to 0.994) with the tibial cross-sectional area. Therefore, tibiofibular synostosis should be performed if necessary because the benefits outweigh the adverse effects. In contrast to common belief, Joseph and Mathew29) and Fern et al.28) showed that fibular surgery is unnecessary when an intramedullary rod is used, and reported union rates of 83% and 100%, respectively, without fibular surgery. However, these authors used a technique of retrograde trans-tarsal intramedullary rod placement across the ankle joint.
Johnston and Birch24) recommended that a fibular osteotomy is necessary to achieve optimal limb alignment and union when the fibula is intact. They also recommended that a hypoplastic or bowed fibula, which is suggestive of pseudarthrosis, should be resected. We hold the view that a fibular osteotomy and shortening may be indicated to accommodate length requirements at the time of acute tibial compression in selected cases, in which healing of the fibular osteotomy site is of little or no concern. Fibular osteotomy, if performed on the dysplastic fibula, can encourage iatrogenic fibular nonunion. The fibula should typically be stabilized by intramedullary fixation using a Kirschner wire or Steinmann pin, in order to accommodate the small diameter of the canal.
One of the most important treatment principles is maximization of the cross-sectional area of healing at the level of the pseudarthrosis because this safeguards against refracture.22) It is reasonable to assume that a wide cross-sectional healing area better resists mechanical stresses. A previous study26) reported that the risk of refracture was significantly higher in patients with a small cross-sectional area. Furthermore, the ratio of the smallest cross-sectional area of healing at the level of the pseudarthrosis relative to the cross-sectional area of the proximal metaphysis of the tibia is a valuable predictor of refracture (unpublished data). In other words, a narrow tibial shaft in the presence of a relatively wide proximal metaphysis increases the risk of refracture, regardless of age. This means that to reduce the risk of refracture, both the ratio of the cross-sectional areas of the metaphysis and shaft of the tibia and the absolute value of the cross-sectional area of the tibial shaft at the pseudarthrosis level should be considered prior to surgery.
In CPT patients associated with NF-1, it was observed that fibrous hamartoma cells maintained some of the mesenchymal lineage phenotype, and did not undergo osteoblastic differentiation in response to BMP. Furthermore, these cells were more osteoclastogenic than normal tibial periosteal cells.33) Based on this observation, it is believed that fibrous hamartoma should be resected completely at the time of osteosynthesis. Two recent reports regarding the use of recombinant human BMPs administered as supplements at the time of pseudarthrosis repair are intriguing. Richards et al.34) reported radiographic union at an average of 33 weeks postoperatively in six of the seven patients who underwent Williams' rodding, BMP-2 supplementation and autogenous iliac bone grafting. In this previous study, a BMP-soaked collagen sponge was wrapped around bone grafts placed circumferentially around pseudarthrosis sites, and a robust callus was observed at the pseudarthrosis sites after the administration of rhBMP-2 without adverse effects. However, Lee et al.35) reported healing failure in four out of five patients treated with external fixation in conjunction with an allograft-BMP-7-collagen carrier complex at the pseudarthrosis sites. In this study, resorption of the complex was observed with time, and the authors concluded that the use of BMP alone may not be sufficient to overcome the poor healing environment associated with CPT. Our stance on this topic is that it may be reasonable to use rhBMPs as an adjunctive osteoinductive biologic material in CPT only after a complete excision of the fibrous hamartoma because fibrous hamartomatous tissue does not respond to BMP signaling. Time will provide an answer to the genuine efficacy of BMPs in CPT.
The Ilizarov technique has been used in our hospital to treat CPT since 1989 with high union rates. However, during the first ten years, the management of co-existing fibular pseudarthrosis was often neglected, and the main focus was placed on tibial osteosynthesis, which we considered to contribute to ankle valgus and resultant refracture. During this period, when hypoplastic fibulae were osteotomized for length adjustment relative to the tibiae at the time of acute compression osteosynthesis, the osteotomized fibulae tended to remain non-united, which apparently contributed to refracture. In January 1999, we started to apply an algorithmic, multi-targeted approach, and paid greater attention to fibular management at the time of tibial osteosynthesis. This resulted in an increase in the refracture-free cumulative survival rate (unpublished data).
The fibular status can be classified into two types (Fig. 1). Type A has normal fibular integrity in the presence of established atrophic-type CPT, whereas type B has atrophic-type CPT with concomitant fibular pseudarthrosis. These types can be subdivided further. Type A1 has a normal appearing fibula or mild dysplasia, whereas type A2 has moderate fibula dysplasia but without fracture or pseudarthrosis. Type B1 has mild dysplasia (> 50% of the width of the contralateral normal side) with little or no proximal migration of the distal fibular physis relative to the tibia. Type B2 shows moderate dysplasia with the atrophic ends, and mild proximal migration of the distal fibular physis relative to the tibia. Type B3 has a severely dysplastic fibula with atrophic ends, which is often associated with a narrow or obliterated medullary canal, and shows marked proximal migration of the distal fibular physis.
In hypertrophic-type CPT, the closed compression or distraction technique may be selectively indicated, whereas in atrophic-type CPT, the treatment strategy depends on the presence or absence of a coexisting fibular pseudarthrosis severity, the locations of dysplastic tibial and fibular pseudarthrosis, and the extent of leg length shortening. Bone transport of the tibia is advocated when no established fibular pseudarthrosis is present (type A) and the resection gap of the tibia is substantial. However, circumferential-onlay bone grafting may be indicated if the resection gap is small. Furthermore, when residual leg length shortening after healing is expected to be < 2-3 cm, fibular osteotomy and shortening may be indicated to accommodate its length at the time of acute compression of the tibia. In this situation, the fibula should be stabilized by intramedullary fixation because of the potential nonunion of the fibula after osteotomy. A '4-in-1 osteosynthesis' may be indicated if a fibular osteotomy is contemplated for a type A2 fibula in the presence of a thin tibial shaft at the level of the pseudarthrosis, as this would increase the cross-sectional area of healing. The term '4-in-1 osteosynthesis' is used to describe the placing of all four proximal and distal segments of the tibia and fibula in one bone healing mass.
In type B with an established fibular pseudarthrosis, it is essential to apply different fibular management strategies, which depend on the subtype. When the quality of the fibula is sufficient (type B1), end-to-end osteosynthesis of the fibula at the time of tibial osteosynthesis is preferred to restore normal ankle mortise. However, when the pseudarthrotic fibula is too dysplastic, all efforts should be made to stabilize the ankle either by using a '4-in-1 osteosynthesis' for type B2 (Fig. 3) or by distal tibiofibular fusion for type B3. Distal tibiofibular fusion (the Langenskiöld method) is indicated only for type B3 or when other techniques have failed. When proximal migration of the fibula is deemed too severe, the fibula can be pulled down to the corresponding level of the distal tibia using the pulling system of the Ilizarov device followed by secondary distal tibiofibular fusion. Therefore, distal tibiofibular fusion is considered primarily for ankle stabilization, whereas '4-in-1 osteosynthesis' is considered for ankle stabilization and union given the large cross-sectional area. Any technique that requires permanent ankle joint transfixation should not be considered as a primary choice. '4-in-1 osteosynthesis' appears to have several advantages because it a) maximizes the cross-sectional area of healing at the pseudarthrosis level, b) facilitates bony healing due to autogenous bone grafting over a wider area, c) provides ankle stabilization and prevents proximal migration of the distal segment of the fibula, which causes ankle valgus, and d) preserves ankle mobility.
When residual shortening > 2-3 cm is anticipated, either a bifocal compression-distraction technique (acute resection, realignment, and compression with proximal lengthening) or internal bone transport is recommended. For bone transport, the docking sites are debrided routinely by excising the interposing fibrous tissue to establish viable bleeding bone ends. The docking site is normally stabilized by cross-pinning in conjunction with copious autogenous bone grafting. Proximal tibial lengthening by distraction osteogenesis can be performed at the metaphyseal, physeal or subphyseal level. Proximal metaphyseal lengthening is indicated when there is no proximal tibial dysplasia and no previous lengthening history. In contrast, chondrodiatasis or subphyseal lengthening may be better indicated when there is obvious proximal tibial dysplasia or a previous lengthening history.36) Furthermore, residual valgus deformities of the ankle and knee may benefit from timely medial hemiepiphysiodesis of the tibia to achieve guided-growth control.
We advocate utilizing a smooth Steinmann pin as an intramedullary rod primarily to help realign the tibial segments during the Ilizarov operation, and minimize the shearing force at the pseudarthrosis site during healing.26) The rod can be removed after bony union when the affected lower leg regains perfect ground mechanical axis alignment in the absence of proximal tibial dysplasia and has a large cross-sectional area of healing mass. Otherwise, we prefer to retain the pin in the tibial medullary canal at the time of the Ilizarov frame removal. Nevertheless, retention of a rod across the previous pseudarthrosis should have biomechanical benefits, even though it cannot prevent the biological process underlying refracture. After healing, a total contact short leg brace is recommended until skeletal maturity because the risk of refracture persists until or after skeletal maturity.
This review reaffirms that the Ilizarov technique is an effective, safe and practical treatment option for managing atrophic-type CPT. In contrast to other treatment modalities, the Ilizarov technique allows multiple targets to be realized, i.e., osteosynthesis, ankle stabilization and leg-length equalization. However, to minimize residual challenges after primary healing, optimum surgical intervention based on the basic treatment principles is essential for obtaining and maintaining union while minimizing a deformity, for maximizing the cross-sectional area of the healing mass, and stabilizing the ankle mortise by fibular stabilization. Hopefully, in the future, combined surgical and medical treatments that effectively modulate or block the biochemical pathways responsible for CPT will downgrade its recalcitrant status.
Figures and Tables
Fig. 1
Classification of fibular status. Type A has normal fibular integrity in the presence of established atrophic-type congenital pseudarthrosis of the tibia (CPT), whereas type B has atrophic-type CPT with concomitant fibular pseudarthrosis. Type A1 has a fibula with a normal appearance or mild dysplasia, and type A2 has moderate fibula dysplasia but without fracture or pseudarthrosis. Type B1 has mild dysplasia (> 50% of the width of the contralateral normal side) with little or no proximal migration of the distal fibular physis relative to the tibia; type B2 shows moderate dysplasia with atrophic ends and mild proximal migration of the distal fibular physis relative to the tibia; whereas type B3 has a severely dysplastic fibula with atrophic ends, which is often associated with a narrow or obliterated medullary canal, and marked proximal migration of distal fibular physis.
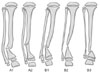
Fig. 2
The author's fibular status-based algorithmic approach for osteosynthesis, ankle stabilization and leg-length equalization. We advocate internal bone transport of the tibia when there is no established fibular pseudarthrosis (type A) and the resection gap of the tibia is substantial. Circumferential-onlay bone grafting may be indicated if the resection gap is small. Fibular osteotomy and shortening may be indicated to accommodate its length at the time of acute compression of the tibia. When the quality of the fibula is sufficient (type B1), end-to-end osteosynthesis of the fibula at the time of tibial osteosynthesis is preferred to restore normal ankle mortise. However, when the pseudarthrotic fibula is too dysplastic, all efforts should be made to stabilize the ankle using either a '4-in-1 osteosynthesis' for type B2 or by distal tibiofibular fusion for type B3. When more than 2-3 cm of residual shortening is anticipated, either the bifocal compression-distraction technique (acute resection, realignment, and compression with proximal lengthening) or internal bone transport is recommended. LLD: limb length discrepancy, BG: bone graft, TL: tibial lengthening, TF: tibiofibular.
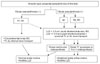
Fig. 3
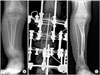
'4-in-1 osteosynthesis' for atrophic-type congenital pseudarthrosis of the tibia (CPT), with type B2 fibular pseudarthrosis. (A) Preoperative radiograph taken at age 6 years and 4 months. (B) Immediate postoperative radiograph showing a combined Ilizarov bifocal, distal compression and proximal distraction method and intramedullary nailing using the smooth Steinmann pin. Approximately 6 cm length gain could be obtained. (C) Radiograph taken at age 9 years showing a well-aligned tibia.
ACKNOWLEDGEMENTS
This investigation was performed at the Department of Orthopedic Surgery, Seoul National University Children's Hospital.
References
1. Boyd HB. Pathology and natural history of congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1982. (166):5–13.

3. Tudisco C, Bollini G, Dungl P, et al. Functional results at the end of skeletal growth in 30 patients affected by congenital pseudoarthrosis of the tibia. J Pediatr Orthop B. 2000. 9(2):94–102.

4. Boero S, Catagni M, Donzelli O, Facchini R, Frediani PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: treatment with Ilizarov's device. J Pediatr Orthop. 1997. 17(5):675–684.

5. Guidera KJ, Raney EM, Ganey T, Albani W, Pugh L, Ogden JA. Ilizarov treatment of congenital pseudarthroses of the tibia. J Pediatr Orthop. 1997. 17(5):668–674.

6. Ghanem I, Damsin JP, Carlioz H. Ilizarov technique in the treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997. 17(5):685–690.

7. Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000. 9(1):3–10.

8. Johnston CE 2nd. Congenital pseudarthrosis of the tibia: results of technical variations in the charnley-williams procedure. J Bone Joint Surg Am. 2002. 84(10):1799–1810.
9. Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia: a long-term follow-up study. J Bone Joint Surg Am. 2004. 86(6):1186–1197.

10. Anderson DJ, Schoenecker PL, Sheridan JJ, Rich MM. Use of an intramedullary rod for the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1992. 74(2):161–168.

11. Kim HW, Weinstein SL. Intramedullary fixation and bone grafting for congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 2002. (405):250–257.

12. Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1990. 72(5):654–662.

13. Pho RW, Levack B, Satku K, Patradul A. Free vascularised fibular graft in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1985. 67(1):64–70.

14. Fabry G, Lammens J, Van Melkebeek J, Stuyck J. Treatment of congenital pseudarthrosis with the Ilizarov technique. J Pediatr Orthop. 1988. 8(1):67–70.

15. Plawecki S, Carpentier E, Lascombes P, Prevot J, Robb JE. Treatment of congenital pseudarthrosis of the tibia by the Ilizarov method. J Pediatr Orthop. 1990. 10(6):786–790.

16. Kristiansen LP, Steen H, Terjesen T. Residual challenges after healing of congenital pseudarthrosis in the tibia. Clin Orthop Relat Res. 2003. (414):228–237.

17. Gracheva VI, Makushin VD, Shevtsov VI, Kuftyrev LM, Degtiarev VE. Ilizarov's transosseous osteosynthesis in treating congenital pseudoarthroses of the leg. Ortop Travmatol Protez. 1981. (7):34–38.
18. Ilizarov GA, Gracheva VI. Bloodless treatment of congenital pseudarthrosis of the crus with simultaneous elimination of shortening using dosed distraction. Ortop Travmatol Protez. 1971. 32(2):42–46.
19. Herring JA. Tachdjian's pediatric orthopaedics. 2008. 4th ed. Philadelphia: Saunders;1007–1022.
20. Vander Have KL, Hensinger RN, Caird M, Johnston C, Farley FA. Congenital pseudarthrosis of the tibia. J Am Acad Orthop Surg. 2008. 16(4):228–236.

21. Toh S, Harata S, Tsubo K, Inoue S, Narita S. Combining free vascularized fibula graft and the Ilizarov external fixator: recent approaches to congenital pseudarthrosis of the tibia. J Reconstr Microsurg. 2001. 17(7):497–508.

22. Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992. (280):81–93.

23. Rajacich N, Bell DF, Armstrong PF. Pediatric applications of the Ilizarov method. Clin Orthop Relat Res. 1992. (280):72–80.

24. Johnston CE, Birch JG. A tale of two tibias: a review of treatment options for congenital pseudarthrosis of the tibia. J Child Orthop. 2008. 2(2):133–149.

25. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994. 25(3):425–465.

26. Cho TJ, Choi IH, Lee SM, et al. Refracture after Ilizarov osteosynthesis in atrophic-type congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2008. 90(4):488–493.

27. Inan M, El Rassi G, Riddle EC, Kumar SJ. Residual deformities following successful initial bone union in congenital pseudoarthrosis of the tibia. J Pediatr Orthop. 2006. 26(3):393–399.

28. Fern ED, Stockley I, Bell MJ. Extending intramedullary rods in congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1990. 72(6):1073–1075.

29. Joseph B, Mathew G. Management of congenital pseudarthrosis of the tibia by excision of the pseudarthrosis, onlay grafting, and intramedullary nailing. J Pediatr Orthop B. 2000. 9(1):16–23.

30. Joseph B, Somaraju VV, Shetty SK. Management of congenital pseudarthrosis of the tibia in children under 3 years of age: effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop. 2003. 23(6):740–746.

31. Grill F, Bollini G, Dungl P, et al. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study: European Paediatric Orthopaedic Society (EPOS). J Pediatr Orthop B. 2000. 9(2):75–89.

32. Morrissy RT, Riseborough EJ, Hall JE. Congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1981. 63(3):367–375.

33. Cho TJ, Seo JB, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg Am. 2008. 90(12):2735–2744.

34. Richards BS, Welch RD, Shrader MW, Johnston CE. Use of rhBMP-2 in congenital pseudarthrosis of the tibia. 2005. In : Annual Meeting of the Pediatric Orthopedic Society of North America; 2005 May 12-15; Ottawa, ON. Rosemont, IL: The Pediatric Orthopedic Society of North America.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download