Abstract
Hansenula anomala (H. anomaly) is part of the normal flora in the alimentary tract and throat. It has been reported to be an organism causing opportunistic infections in immunocompromised patients. However, cases of fungal arthritis caused by H. anomala are rare. We encountered a case of H. anomala arthritis in a 70-year-old man who was treated with an empirical antibiotic treatment and surgery under the impression of septic arthritis. However, the patient did not improve after antibiotic therapy and surgery. Consequently, knee joint aspiration was performed again, which identified fungal arthritis caused by H. anomala. It was treated successfully with amphotericin B and fluconazole. When treating arthritis patients with diabetes, it is important to consider the possibility of septic arthritis by H. anomala and provide the appropriate treatment.
Hansenula anomala is an ascosporogenous yeast of the ascomycetes class. Only 2 genera of this species, Hansenula anomala and Hansenula polymorphia, are known to cause disease in humans. Pichia anomala is a new name and a synonym for Hansenula anomala. Hansenula anomala is a nonpathogenic fungus found in plants, soil, and fruits and is indigenous in the alimentary and respiratory tracts of animals and humans.1)
Human infections caused by Hansenula anomala, an opportunistic fungus, occur in new born infants, premature infants, and immunodeficient patients but are rare. There are reported cases of pneumonia, infective endocarditis, urinary tract infections, and oral mucosa infections caused by fungemia in immunocompromised patients.1-4) These cases are also rare but there has been an increase in incidence recently.4) Domestically, Park et al.4) reported a case of pneumonia caused by fungemia. We report a case of fungal arthritis due to Hansenula anomala in a diabetic patient at our institution.
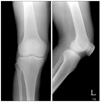
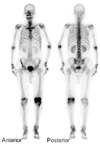
A 70-year-old male was admitted to our hospital primarily due to pain and swelling of the left knee that had been present for more than 1 month. He had no specific diseases except for diabetes and was transferred to our institution due to signs of inflammation observed in a fine needle aspiration biopsy performed at a nearby hospital more than 1 month prior to admission. Based on the history of the illness, oral medication was administered to treat the type II diabetes. The patient's vital signs at the time of admission were as follows: blood pressure 130/80 mmHg, pulse rate 95 bpm, respiratory rate 20 breaths/min, and temperature 36.8℃. In the physical examination, severe swelling of the left knee, minor flare, and local thermal sensations were found as well as passive movements caused severe pain. In the blood test performed upon admission, the white blood cell count, hemoglobin, platelet count, erythrocyte sedimentation rate and C-reactive protein was 18,500/mm3, 11 g/dL, 286,000/mm3, 34 mm/hr and 2.4 mg/dL, respectively. The fine needle aspiration biopsy revealed reddish-brown exudates. Joint fluid analysis showed a red blood cell count, white blood cell count and a glucose level of 4,400/mm3, 84,560/mm3 (neutrophil 80%, lymphocyte 10%, and monocyte 10%) and 228 mg/dL, respectively, and no nodes were observed. No microorganisms were found in the bacterial culture. On the plain radiographs, minor joint space narrowing was observed in the medial compartment of the left knee but no abnormal findings were noted (Fig. 1). A bone scan showed increased uptake in the left knee (Fig. 2).
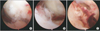
Septic arthritis was suspected based on these findings and an arthroscopic synovectomy and lavage were performed on the 2nd day of admission (Fig. 3). During surgery, severe synovitis and massive sphacelus were noted. The pathology examination of the sphacelus revealed non-specific inflammation as well as the deposition of fibrin and hemosiderin.
A fine needle aspiration biopsy performed on the day of admission and a bacterial culture carried out during surgery did not reveal any microorganisms. Considering that the antibiotic treatment performed at another hospital resulted in such outcomes, the antibiotic treatment was continued after surgery with the assumed diagnosis of septic arthritis. However, the knee pain did not disappear even 1 month after surgery and the blood test results showed a postoperative increase in the erythrocyte sedimentation rate and C-reactive protein level. Therefore, a reoperation was required.
In the 2nd surgery, an arthroscopic synovectomy and lavage were carried out due to signs of synovitis but bacteria were identified. The 2nd operation did not lead to an improvement in the patient's condition and the erythrocyte sedimentation rate and C-reactive protein remained high. Accordingly, a fine needle aspiration biopsy was performed again 3 weeks after the 2nd operation. An organism suspected to be yeast was found in the bacterial culture, which led to an additional fungus culture that identified Hansenula anomala.
A single dose of IV amphotericin B (500 mg daily) was administered to the patient for 8 weeks. However, the treatment was interrupted due to an increase in the blood urea nitrogen and creatine caused by the nephrotoxicity of amphotericin B. Therefore, it was replaced with another antifungal agent, fluconazole. The patient was discharged when the symptoms improved and the erythrocyte sedimentation rate and C-reactive protein became normal after 7 weeks of an intravenous injection of a daily dose of 400 mg of fluconazole. Oral fluconazole treatment was prescribed for 6 weeks after discharge. No signs of relapse were identified until the 6th postoperative month and the patient is currently being followed up on an outpatient basis.
Contrary to the assumptions so far, many reports have shed light on the pathogenicity of fungi. Among them, Candida species are the most common cause of human infections, and fungal infections are on the rise. The past decades have witnessed the development of treatments that have improved the survival rate of patients with low immunity due to malignant tumors, premature birth, and organ transplants. These patients are more vulnerable to fungal infections considering that they are immunocompromised and require lengthy hospitalization, intravascular cannulation and extensive antibiotic treatment.
Fungal infections are a major cause of death and disease in immunocompromised patients. The most common strains include Candida, Aspegillus, and Phycomycetes species. As Choi and Choi2) reported in 1996, Hansenula anomala is an opportunistic fungus in immunodepressed patients.
Hansenula anomala was first introduced by Hansen in 1981 and interstitial pneumonia found in a newborn infant by Csillag et al. in 1953 was the first case of human infection caused by Hansenula anomala. In 1958, Wang and Schwarz et al. succeeded in identifying this fungus in the lung.5) According to Taylor et al.,6) Hansenula anomala accounts for 1% of the causes of the pathogenic fungaemia.
The presumed predisposing factors and underlying diseases of Hansenula anomala infections include low birth weight, drug addiction, AIDS, cancer, total parenteral nutrition, and the extensive use of antibiotics. Human infections caused by Hansenula anomala are rare and present in a range of patterns. It can cause severe infections, such as sepsis, pneumonia, endocarditis, and ventriculitis, in patients with hematologic malignancy and immunodepression as well as in premature infants.1-4) According to Choi and Choi2) fungemia caused death in 64% of infected patients. Hansenula anomala infections in patients with a normal immune system are uncommon and cases of fungal arthritis caused by Hansenula anomala are even more rare.
The most common cause of fungal infections of the joint is a direct injection into the joint, such as intraarticular injection and surgery. Infections caused by hematogenous dissemination are prevalent in those addicted to drugs. According to a report on fungal arthritis in 8 patients by Vicari et al.7) in 2003, severe leukocytopenia and the use of immunosuppressants and intravascular cannulation can be predisposing factors. Therefore, it is believed that a fungal culture test should accompany a fine needle aspiration biopsy and intraoperative bacterial culture, which are performed under the suspicion of pyogenic arthritis in patients with these risk factors. In addition, a fungal infection should be considered when the condition becomes chronic without identification of the bacteria or an adequate response to antibiotics and surgery, as in our case.
The patient described in this report was normally healthy and had no risk factors for a fungal infection but was under treatment for diabetes. Therefore, it is believed that the occurrence of Hansenula anomala infection in the knee was caused by the trait of the fungus growing well in a high-sugar-containing medium rather than by immunodepression.8) In addition, the use of intraarticular injections due to degenerative arthritis performed at another institution prior to the development of the fungal infection might be a possible cause.
Diabetic patients are more vulnerable to fungal infections and less responsive to treatment than others. Accordingly, diabetes should be considered one of the risk factors for fungal infections. In addition, considering that an intraarticular injection is the preferred treatment option for degenerative arthritis in Korea, the patient's record of illness documented at other institutions should be monitored closely.
Although the proper treatment procedures for Hansenula anomala infections have yet to be established, some reports have described it as being susceptible to amphotericin B, flucytosine, and fluconazole.9) In this patient, 8 days use of amphotericin B led to changes in the erythrocyte sedimentation rate and C-reactive protein from 57 mm/hr and 0.25 mg/dL to 38 mm/hr and 0.2 mg/dL, respectively. Therefore, Hansenula anomala was receptive to amphotericin B, even though the antifugal agent had to be replaced by fluconazole due to adverse drug reactions, such as increased blood urea nitrogen and creatine. After 7 weeks of IV Fluconazole therapy, the erythrocyte sedimentation rate and C-reactive protein became normal confirming its efficacy.
According to Cuellar et al.,10) fungal arthritis can be treated with the proper use of antimicrobial agents and surgery, even though adjustments should be made depending on the species. It is believed that surgical treatments, such as drainage and synovectomy, accompanying the use of antimicrobial agents are important factors for the treatment of fungal arthritis, and amputation may be required for resistant bacteria and life-threatening fungemia nonresponsive to these treatments.
In many cases, fungal arthritis, unlike other infections, is difficult to diagnose early due to the difficulty in identification, resulting in a longer duration of illness and chronic status. A diagnostic delay also occurred in our case because the culture test was not performed during the initial examination and 1st operation, and the test performed during the 2nd operation did not indicate a fungal infection. It is possible that the fungal infections might have occurred during surgery or treatment. However, it can be concluded that a fungal infection is the cause of the arthritis considering that no notable change was observed in the patient's clinical condition after surgery and treatment and that the antifungal agent treatment resulted in an improvement in symptoms and normalization of the erythrocyte sedimentation rate and C-reactive protein. As shown in our case, important factors for the treatment of fungal arthritis are early diagnosis and the proper use of antifungal agents.
We report a case of fungal arthritis caused by Hansenula anomala in a diabetic patient treated by surgery and antifungal agents.
Figures and Tables
References
1. Haron E, Anaissie E, Dumphy F, McCreie K, Fainstein V. Hansenula anomala fungemia. Rev Infect Dis. 1988. 10(6):1182–1186.

2. Choi HS, Choi TY. Analysis of 22 cases of Fungemia due to Hansenula anomala. Korean J Clin Pathol. 1996. 16(2):201–210.
3. Murphy N, Buchanan CR, Damjanovic V, Whitaker R, Hart CA, Cooke RW. Infection and colonisation of neonates by Hansenula anomala. Lancet. 1986. 1(8476):291–293.

4. Park SS, Lee SH, Lee MA, Hong KS. Hansenula anomala pneumonia in a patient with lung cancer. Korean J Clin Pathol. 1994. 14(4):463–469.
5. Ma JS, Chen PY, Chen CH, Chi CS. Neonatal fungemia caused by Hansenula anomala: a case report. J Microbiol Immunol Infect. 2000. 33(4):267–270.
6. Taylor GD, Buchanan-Chell M, Kirkland T, McKenzie M, Wiens R. Trends and sources of nosocomial fungaemia. Mycoses. 1994. 37(5-6):187–190.

7. Vicari P, Feitosa Pinheiro R, Chauffaille Mde L, Yamamoto M, Figueiredo MS. Septic arthritis as the first sign of Candida tropicalis fungaemia in an acute lymphoid leukemia patient. Braz J Infect Dis. 2003. 7(6):426–428.

8. Klein JJ, Watanakunakorn C. Hospital-acquired fungemia: its natural course and clinical significance. Am J Med. 1979. 67(1):51–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download