Abstract
Background
This review evaluated the safety and efficacy of etanercept in patients with ankylosing spondylitis (AS).
Methods
Of 59 patients with AS, this study reviewed 11 patients who were refractory to conventional therapy and treated with etanercept from September 2005 to January 2008. The mean follow-up duration was 13.6 months. The general improvement was evaluated by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and adverse effects, complications and inflammatory markers were also assessed.
Results
The mean BASDAI decreased from 7.1 ± 1.6 before treatment to 4.2 ± 1.8 at 3 months after the etanercept treatment (p = 0.001). The mean erythrocyte sedimentation rate and C-reactive protein were decreased significantly by the etanercept treatment. The greatest improvement in symptoms was enthesitis, followed by skin involvement and morning stiffness. There was a significant difference in the improvement in BASDAI along with the follow up duration (p = 0.04). A serious infection was observed as a complication in 1 case.
Conclusions
These results suggest that etanercept can induce significant improvement in most patients with less damage. A trial of tumor necrosis factor inhibition is indicated in all AS patients who do not achieve adequate disease control with disease-modifying antirheumatic drugs, such as methotrexate, leflunomide etc. The patients treated with etanercept should be educated about the possibility of infection and monitored closely.
Ankylosing spondylitis (AS), seronegative spondyloarthritis involving primarily the spine and the sacroiliac joint, is a chronic inflammatory rheumatoid disease causing more than 3 months of constant pain.1-4) It is found in 0.5-1% of the population and is 2-3 times more prevalent in males than females.5) AS is associated with inflammation of the axial skeleton, peripheral arthritis, enthesitis and mucocutaneous lesions.3,5) The most common early symptoms include minor and obscure lumbar pain and morning stiffness lasting for more than 3 hours. Such inflammatory back pain can be differentiated from mechanical back pain in that the former increases progressively before the age of 40, lasts for more than 3 months, is associated with morning stiffness, and improves with exercise. Although the factors affecting the progress of AS have not been established, advanced AS can be associated with severe functional impairment, work disability, and a compromised quality of life.1) Therefore, the treatment efforts should be focused on reducing the level of pain, stiffness and fatigue as well as maintaining good posture and physical, psychological, and social functions.1) The treatment methods for AS are strongly dependent on the use of non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, and oral steroids. They are effective in improving symptoms but are ineffective in retarding the progress of the disease. In addition, disease-modifying antirheumatic drugs (DMARDs), such as sulfasalazine or methotrexate, which are used in patients unresponsive to NSAIDs, reduce the peripheral symptoms albeit limitedly but are less effective in the treatment of the symptoms of the axial skeleton.5) Recently, the Assessment in the AS international working group (ASAS) and the European League Against Rheumatism (EULAR) recommended anti-tumor necrosis factor (TNF)-α treatments in AS patients unresponsive to other drugs. There are also reports showing the cost-effectiveness of anti-TNF-α therapies: the treatment is expensive but results in rapid and constant improvement. On the other hand, the US FDA recommended the careful use of etanercept to avoid serious infections. Domestically, etanercept has been used in some patients with AS since 2003. However, its efficacy and safety have not been addressed. This study examined the efficacy, use, and safety of etanercept in patients with ankylosing spondylitis.
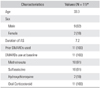
Between September 2005 and February 2008, 59 patients were diagnosed with AS and met the modified New York criteria at our institution. Of these patients, 11 patients, who were unresponsive to conventional drug treatments, were enrolled in this study (Table 1). They had serious active AS showing less than satisfactory changes after more than 3 months use of 2 or more NSAIDs or DMARDs or had side effects requiring discontinuation of therapy.
Etanercept is a fusion protein consisting of two p75 TNF receptor extracellular domains and the Fc portion of human immunoglobin G1 (IgG1). It binds TNF-α selectively, and blocks the interaction of TNF-α with its cell surface receptors. It was approved by the US FDA in November 1998 as the first biological agent for the treatment of rheumatoid arthritis. In Korea, it has been used to treat rheumatoid arthritis from December 2003 since its approval by the Korean FDA in October 2003. As of July 1 2007, it can be prescribed for an additional 6 months if the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score is reduced to 50% of the pre-treatment value or by more than 2 units at the 3rd month. Thereafter, it can be used for up to 24 months if the value obtained at the 3rd month is maintained in the evaluation performed every 6 months.
In this study, 25 mg of etanercept (Enbrel®; Immunex, Seattle, WA, USA) was injected subcutaneously into the patients twice a week with a 72 to 96 hour interval. The patient's data, including gender, age, duration of illness, main complaints, extraarticular symptoms, dosage and duration of treatment with the anti-TNF agent, and use of the conventional agents for the treatment of ankylosing spondylitis, was collected retrospectively from the medical records and by a telephone interview. All the patients were examined for any history of pneumonia prior to the injection and underwent sputum Acid Fast Bacilli (AFB) Stain 3 times, a chest X-ray, a hepatitis B virus test (HBsAg, HBsAb, HBe Ag, HBeAb, HBV DNA), an alanine aminotransferase (ALT) test, and an aspartate aminotransferase (AST) test. The presence of HLA-B27 was determined and the creatinine, bilirubin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and Anti-Nuclear Antibody (ANA) levels were measured after each injection. After the etanercept injection, the occurrence of side effects and complications were examined. The BASDAI was used to assess the level of improvement. Statistical analysis was performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A paired t-test was used to compare the ESR and CRP levels before and after the etanercept injection, and the Kruskal-Wallis test and Mann-Whitney test were employed to compare the BASDAI, ESR, and CRP levels according to the illness duration. A p-value of < 0.05 was considered significant.
The medical records of the 11 patients (9 males and 2 females) with AS were examined. The mean age was 33.3 years (range, 19 to 52 years). The mean duration from the diagnosis of AS to the etanercept treatment was 7.2 years (range, 0.5 to 18 years). The mean follow-up period was 13.6 months (range, 4 to 30 months).
The DMARDs used before the etanercept treatment included methotrexate in 10 (91%) cases, sulfasalazine in 10 (91%) cases, oral corticosteroid in 11 (100%) cases, and hydroxychloroquine in 2 (18%) cases. Etanercept was used for 12, 36, and 72 weeks in 9, 1, and 1 patient, respectively.
All the patients tested positive for HLA-B27. The main complaints at the initial examination were back pain, motor dysfunction and morning stiffness of the spine. Other symptoms included hip joint pain in 2 cases, shoulder pain in 1 case, and knee joint pain in 1 case. Uveitis was the only extraarticular symptom found in 1 case. There were 3 cases of extra-axial arthritis, including 2 cases of coxarthritis and 1 case of gonarthritis. Of the patients, a 22-year-old male with morning stiffness of the lumbar spine and pain in the left hip caused by ankylosing arthritis had been treated with NSAIDs and DMARDs for 1 year. However, the continuing symptoms required total hip arthroplasty and the use of etanercept at the 4th postoperative year. A 19-year old male with pain in his right knee had been treated with NSAIDs and DMARDs for 1 year. However, arthroscopic debridement and synovectomy performed twice due to constant pain and swelling, and etanercept was administered at the 2nd postoperative year.
With regard to the underlying conditions, cardiovascular disorders associated with blood pressure and arrhythmia were encountered in 2 cases, anemia in 1 case, diabetes in 1 case, pneumonia in 1 case, and a tumor in 1 case. A history of pneumonia was found in 1 of the 11 patients, who had achieved full recovery with 1-year use of antituberculosis drug 2 years before the administration of etanercept. The anti-TNF-α therapy was started when there were no signs of active pneumonia according to plain radiography and a sputum examination. In 1 case, a tumor resection was required before the use of etanercept due to multiple osteochondroma, and no relapse or neoplasm was encountered during the therapy.
Before the etanercept treatment, the mean BASDAI was 7.1 ± 1.6 (fatigue, 7.6 ± 1.5; symptoms of the axial skeleton, 7.7 ± 1.6; peripheral arthritis, 6 ± 3.6; enthesitis or skin involvement, 7.1 ± 2.6; level of morning stiffness, 8 ± 2; duration of morning stiffness, 5.3 ± 3.7). The mean BASDAI decreased by 45% to 4.2 ± 1.8 at the 3rd month of the etanercept treatment. Fatigue was reduced by 30% to 5.2 ± 1.5 on average, the symptoms of the axial skeleton improved by 36% to 4.6 ± 1.5, and the level of peripheral arthritis decreased by 43% to 3.2 ± 3.5. The symptoms associated with enthesitis or skin involvement were reduced by 49% to 3.3 ± 2.6. The mean level of morning stiffness decreased by 40% to 4.9 ± 2 and the mean duration of morning stiffness was shortened by 51% to 3.3 ± 2.6. Enthesitis, skin involvement, and morning stiffness showed most improvement since the use of etanercept (Fig. 1). The mean BASDAI was decreased 45% from 7.1 ± 1.6 to 4.2 ± 1.8 using etanercept (p = 0.001).
In 3 patients with a more than 8 year duration of illness, the mean BASDAI decreased from 6.8 ± 0.3 to 4.1 ± 2.7. The mean BASDAI in 5 patients with a 3 to 8 year and a ≤ 3 year duration of illness decreased from 7.5 ± 1.8 to 4.6 ± 1.9 and from 6.6 ± 2.2 to 3.4 ± 1.5, respectively. With regard to the changes in the BASDAI between before the injection and at 3rd months since the injection, marginal statistical significant was identified in those with a 3-8 year duration of illness (p = 0.04), while no statistical significance was found in those with an illness duration ≥ 8 years (p = 0.376) or ≤ 3 years (p = 0.513).
The ESR of the 11 patients decreased from 44.6 ± 34.1 at the time of injection to 19.6 ± 14.1, 20.8 ± 21.9, and 15.8 ± 24.5 at 3, 6, and 12 months since the injection, respectively (Fig. 2). The CRP also fell from 4.1 ± 3.8 at the time of injection, to 1.3 ± 2.3, 1.7 ± 2.9, and 1.8 ± 4.6 at 3, 6, and 12 months since the injection, respectively (Fig. 3). The p-value for the changes in ESR was 0.051, which is slightly higher than 0.05, but still showing marginal significance. On the other hand, the p-value for the CRP changes was 0.045, which showed a significant decrease.
In the 3 patients with a ≥ 8 year duration of illness, the mean ESR decreased from 43.3 ± 20.8 to 24.3 ± 22, 15 ± 11.4, 15 ± 11.4 at 3, 6, and 12 months since the injection, respectively. In the 5 patients with a 3-8 year duration of illness, the mean ESR was 41.8 ± 28.3 before the injection, and 22.4 ± 7.7, 21.3 ± 11.6, and 22.2 ± 35.9 at 3rd, 6th, and 12th months since the injection, respectively. In the 3 patients with ≤ 3 year duration of illness, the mean ESR fell from 50.7 ± 60.7 before the injection to 10.3 ± 14.4, 26 ± 41.6, and 6 ± 6.9 at 3, 6, and 12 months since the injection, respectively. When the ESR levels before the injection and at 12 months since the injection were compared in each patient group categorized according to the illness duration, there was no significant difference between the three groups: ≥ 8 years of duration of illness (p = 0.184), 3-8 years of duration of illness (p = 0.249), and ≤ 3 years of duration of illness (p = 0.121).
The mean CRP decreased from 6 ± 5.1 before the injection to 2.6 ± 4.4, 0.1 ± 0, and 0.7 ± 1.1 at 3, 6, and 12 months since the injection, respectively, in 3 patients with ≥ 8 years of duration of illness; from 4.2 ± 4 before the injection to 0.7 ± 0.6, 1.5 ± 2, and 3.4 ± 6.8 at 3, 6, and 12 months since the injection, respectively, in 5 patients with 3-8 years of duration of illness; and from 2.2 ± 1.9 before the injection to 0.9 ± 1.4, 2.9 ± 4.9, and 0.1 ± 0 at 3, 6, and 12 months since the injection, respectively, in 3 patients with ≤ 3 years of duration of illness. When the CRP levels before the injection and at the 12th month since the injection were compared in each patient group categorized according to the duration of illness, there was no statistical difference between the three groups; patients with ≥ 8 years of duration of illness (p = 0.121), 3-8 years of duration of illness (p = 0.389), and ≤ 3 years of duration of illness (p = 0.121).
A case of pruritus at the injection site developed during the etanercept treatment and a case of superficial infection at the injection site was encountered after the treatment. In one 22-year-old male patient who had undergone total hip arthroplasty due to ankylosis of the left hip 4 years before the etanercept treatment, flare and burning sensation appeared in the left inguinal area after the injection. Three weeks later, the injection was stopped and drainage and biopsy of the infected area were performed urgently. He has been followed since because revision total hip arthroplasty might be necessary. In 1 case, an elevation of the AST/ALT ratio was noted temporarily after the injection, but it was minor and did not develop into fulminant hepatitis or liver cirrhosis. During treatment, an upper respiratory infection was identified in 1 patient in whom the treatment was stopped because the signs of pneumonia were identified from a chest X-ray taken at the 3rd month since the injection. Etanercept was readministered 1 month later when pneumonia was ruled with chest X-ray showing improvement in the lesions and negative sputum examination results.
Back pain in AS is defined as slowly progressive pain worsening during rest, which improves during exercise, and lasts ≥ 3 months. It is considered to be the main symptom of inflammatory spinal disorders and is a key factor for early diagnosis.1) The clinical characteristics of AS can be subcategorized into articular and extraarticular symptoms. The articular symptoms include inflammatory back pain that progresses slowly and sacroilitis. Opisthotonos appears in the later stages and the peripheral joints involved are usually the hip joint and knee joint with rare cases of the upper and lower extremity joints. The extraarticular symptoms include acute anterior uveitis, cardiovascular symptoms, pulmonary involvement, inflammatory intestinal diseases, and osteoporosis. The main symptoms complained of by the patients in the first medical examination in this study were back pain in most cases and hip joint pain or knee joint pain in some cases. With regard to the peripheral joint involvement, there were 3 cases (27%) of hip joint or knee joint involvement. The clinical course of AS vary and include episodes of natural improvement and worsening, which results in mild disability in most cases but severe disability in some cases.6)
Thus far, the treatment of AS has focused on dealing with the symptoms using NSAIDs and kinesitherapy. However, with the recent introduction of anti-TNF a gents, such as etanercept and infliximab, a new chapter has been opened for the treatment of rheumatoid arthritis and AS, and there are many reports on their efficacy and safety based on randomized controlled studies.4,5,7) Still, there is some controversy regarding suitable candidates for treatment, time of the injection, treatment duration and its efficacy in preventing the progress of rheumatoid arthritis and AS.4,7-9) The US FDA reported in March 2008 that etanercept treatments led to serious infections requiring hospital stays or caused life-threatening conditions. They recommended that patients be monitored for symptoms or signs of infection during treatment.10) Complications, such as tumors and autoimmune diseases, can also occur. In addition, it is important to select suitable candidates who would be expected to be responsive to the treatments because these anti-TNF agents are expensive.
Clinicians using anti-TNF agents should be well aware of the possible side effects and perform precise assessments. One of the most important side effects is an increased risk of severe infections, and the anti-TNF agents should be used with extreme care in patients vulnerable to infections, such as diabetics. Patients given anti-TNF treatments should be provided with explanations on the risk of infections and admonished not to ignore the signs of a serious infection and reported them to their doctors as soon as possible. In addition, when a surgical procedure is required in a patient under an anti-TNF treatment, careful caution should be taken before and after surgery, and the agent should be re-administered after recovery of the surgical site is obtained.11) In this study, the patient with previous total hip arthroplasty reported a superficial infection in the left inguinal area 3 weeks after its occurrence. Accordingly, early treatment was delayed and revision surgery is under consideration because a deep infection around the implant is suspected. Therefore, it is believed that a more careful follow-up and treatment are needed for patients with implants.
Local flare and swelling associated with a subcutaneous injection of the agents are the most common side effects, which usually improve within 24 hours. These conditions can be treated with antihistamine without the need to stop the anti-TNF agents because they have no impact on the efficacy of the agents used.5) In this study, a case of minor pruritus was observed after the injection.
The treatment results of AS can be assessed in terms of the quality of life using the Ankylosing Spondylitis Quality of Life Questionnaire, of the disease activity using the BASDAI, and of the functions and joint stiffness using the Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Metrology Index. In this study, the BASDAI was used to assess the disease activity in patients under the etanercept treatment and significant improvement in the patients' conditions was observed after 12 weeks of the injection. With regard to the decrease in the BASDAI in patients subdivided according to the illness duration, remarkable improvement was found in all patients.
The ESR is a nonspecific test that is determined by the concentration of plasma proteins, particularly fibrinogen, and is affected by other factors, such as age, gender, and time of measurement. In contrast, the CRP level increases in response to tissue damage, rises continuously due to chronic inflammation, and is less affected by other factors. In this study, changes in the ESR and CRP levels were observed to assess the improvement in chronic inflammation. The ESR decreased drastically at 3 months since the injection and by 64% at 12 months since the injection compared to the value measured before the injection. The CRP also decreased the most at 3 months since the injection and by 57% at 12 months since the injection. Each reduction ratio of the ESR and CRP at 12 months since the injection was analyzed, but there was no significant difference between the groups divided according to the illness duration. This suggests that the inflammatory response to the etanercept injection decreased the most at 3 months since the injection and continued to fall until 12 months since the injection. There are many reports on the prompt and prolonged effect of the biological agents.2-5) However, there are still many unanswered questions: whether the long-term use of these biological agents can be effective in reducing inflammation; whether the use of such agents can result in radiological improvement; whether their efficacy can be maintained; and whether the injection should be stopped or continued based on certain standards.
The etanercept injection resulted in a reduced inflammatory response and improvement in the BASDAI scores in patients with AS who were unresponsive to their previous treatments. It is believed that etanercept is effective in blocking the progress of the disease and improving the symptoms in AS patients who are unresponsive to DMARDs, such as methotrexate and leflunomide, despite the risk of side effects and complications. However, considering the risk of infection during injection, it should be used with extreme care in patients vulnerable to infection. Patients under etanercept treatment should be monitored closely and alerted to the risk of infection. Future studies should focus on determining the long-term efficacy of biological agents and the standards to which treatment decisions, such as continuation/discontinuation of the treatment and injection time, are based using a long-term follow-up and an examination of various parameters.
Figures and Tables
Fig. 1
Mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores of the patients who received etanercept at wk 0, 12. The mean BASDAI scores decreased from the baseline (p = 0.001).

Fig. 2
Disease activity indicator (erythrocyte sedimentation rate [ESR]); ESR at 0, 3, 6, 12 mo decreased significantly (p = 0.051).

Fig. 3
Disease activity indicator C-reactive protein (CRP); CRP at 0, 3, 6, 12 mo decreased significantly (p = 0.045).

References
1. Braun J, Brandt J, Listing J, Rudwaleit M, Sieper J. Biologic therapies in the spondyloarthritis: new opportunities, new challenges. Curr Opin Rheumatol. 2003. 15(4):394–407.

2. Braun J, de Keyser F, Brandt J, Mielants H, Sieper J, Veys E. New treatment options in spondyloarthropathies: increasing evidence for significant efficacy of anti-tumor necrosis factor therapy. Curr Opin Rheumatol. 2001. 13(4):245–249.

3. Davis JC Jr, van der Heijde DM, Braun J, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis. 2008. 67(3):346–352.

4. van Eijk IC, Peters MJ, Serne EH, et al. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockade. Ann Rheum Dis. 2009. 68(3):362–366.

5. Reed MR, Taylor AL. Tumor necrosis factor inhibitors in ankylosing spondylitis. Intern Med J. 2008. 38(10):781–789.
6. Liu Y, Cortinovis D, Stone MA. Recent advances in the treatment of the spondyloarthropathies. Curr Opin Rheumatol. 2004. 16(4):357–365.

7. Song JS. Review of tumor necrosis factor inhibitors on rheumatoid arthritis. J Korean Rheum Assoc. 2007. 14(1):1–14.

8. Chang J, Girgis L. Clinical use of anti-TNF-alpha biological agents: a guide for GPs. Aust Fam Physician. 2007. 36(12):1035–1038.
9. Yun HR, Kim TJ, Kim TH, Choi HS, Bae SC. Anti-TNF-alpha therapy in rheumatic diseases with chronic hepatitis B virus infection. J Korean Rheum Assoc. 2007. 14(3):242–250.

10. Her MY, Sheen D, Kim TH. Treatment of ankylosing spondylitis. J Korean Rheum Assoc. 2006. 13(1):1–9.
11. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006. 14(9):544–551.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download