Abstract
Background
Patients with ankylosing spondylitis (AS) achieve early bone union compared to those with other spinal diseases. This study compared the time to bone union after surgery between AS patients and degenerative spinal disease patients.
Methods
Patients with degenerative spinal diseases (control group) and AS (experimental group) underwent pedicle subtraction osteotomy followed by posterolateral fusion, and decompression and posterolateral fusion, respectively. There were 10 patients in the experimental group. The control group included 26 patients who were less than 50 years of age and underwent two-level autogenous grafting after decompression and spinal fusion. Autogenous grafts and a range of bone substitutes were used in the experimental group, whereas only autogenous grafts were used in the control group. Bone union was determined on the radiographs and 3-dimensional CT scan images. The level of union was assessed using the Lenke's and Christensen's classification systems.
Results
In the experimental group, the mean age was 41.3 years (range, 30 to 67 years), the mean follow-up period was 21.7 months (range, 12 to 43 months), and bone union was confirmed at an average of 3.5 months (range, 3 to 5 months) after surgery. In the control group, the mean age was 43.1 years (range, 35 to 50 years), the mean follow-up period was 21.8 months (range, 12 to 74 months), and bone union was observed at an average of 5.6 months (range, 4 to 12 months) after surgery. The difference in the time to bone union between the two groups was significant (p = 0.023).
Ankylosing spondylitis (AS) is a disease that involves the sacroiliac joint and all the way up to the upper spine. It is characterized by reduced joint motion due to calcification of the spinal ligaments and joint capsules, and it can be accompanied by kyphosis because of changes in either lumbar lordosis or thoracic kyphosis.1,2) Patients with AS experience significant restrictions in their daily activities. In particular, when the disease affects the cervical spine, patients can neither see straight ahead in the standing position nor lie on their back, which disrupts their daily and social activities, necessitating surgical intervention.3,4)
Osteotomy, pedicle screw fixation and bone grafting are popular treatments for kyphosis. The long-term prognostic factors of surgery include the type of osteotomy, the degree of angular correction, and the union of grafted bone. However, there are no reports of nonunion after surgery in AS.5-7) Clinicians are aware through experience that the union of grafted bones is achieved earlier in patients with AS than in those with other spinal diseases. This retrospective study compare the interval from surgical treatment to the union of grafted bone between patients with AS and those with degenerative spinal diseases.
Of the AS patients with kyphotic deformity who had been admitted to our institution between January 2003 and January 2009, 10 patients who had been treated with posterolateral fixation after the osteotomy and bone grafting were included in the experimental group. The control group contained 26 patients who had been treated with decompression and posterolateral fusion and bone grafting for degenerative spinal diseases. Nine of the 10 patients with AS underwent pedicle subtraction osteotomy. The remaining patient underwent Smith-Petersen osteotomy. Bone grafting included the two levels adjacent to the osteotomized site. The inclusion criteria for the control group were less than 50 years of age, posterolateral fusion and two-level grafting considering that AS is common in young people and two-level grafting had been performed in the experimental group. In the control group, there were 15, 8, and 3 cases of spinal stenosis, spondylolisthesis, and intervertebral disc herniation, respectively.
Bone grafting was performed with autogenous bone grafts collected during the osteotomy and bone substitutes in the experimental group, whereas it was performed with autogenous bone grafts collected during decompression and autogenous iliac bone grafts in the control group. Bone substitutes were used in 9 cases in the experimental group, (a composite of hydroxyapatite and calcium phosphate in 6, demineralized bone matrix in 2, and an allograft in 1).
Bone union was assessed from the radiographs (anteroposterior, lateral, and oblique views) taken before surgery, after surgery and at regular intervals during the follow-up period. The radiographic results were evaluated using the Lenke's classification system8)
to assess the overall union, and the Christensen's classification system9) to examine the intersegmental union. Bone union was considered to have occurred when type A union (solid bilateral union) and type B union (unilateral large fusion mass with contralateral small fusion mass) according to the Lenke's system or grade 3 union (presence of a bony bridge) according to the Christensen's system were observed on the radiographs. Three-dimensional CT was also performed when bone union was not evident on the radiographs.
The statistical analysis for a comparison of the time to bone union between the two groups was performed using a Mann-Whitney test.
In the experimental group, there were 7 males and 3 females, and the number of fused segments was 40. Their mean age and follow-up period was 41.3 ± 10.7 years (range, 30 to 67 years) and 21.7 months (range, 12 to 43 months), respectively. In the control group, there were 12 males and 14 females, and the number of the fused segments was 104. Their mean age and follow-up period was 43.1 ± 7.4 years (range, 35 to 50 years) and 21.8 months (range, 12 to 74 months), respectively (Table 1). There were no complications, such as infections and nonunion, after surgery in both groups.
According to the Christensen's classification, grade 3 bone union was achieved in 60% and 100 % of the 40 levels in the experimental group at 3 months and 4 months after surgery, respectively. In the control group, grade 3 bone union was obtained in 50% and 100% of the 104 levels at 5 and 12 months after surgery, respectively (Fig. 1). Bone union could not be assessed using the radiographs alone in 3 cases of the experimental group and 5 cases of the control group. Union could be confirmed with CT in 4 segments in the experimental group and 7 segments in the control group, which were grade 1 or 2 union according to the Christensen's classification system (Fig. 2).
Bone union according to the Lenke's classification system was observed at an average of 3.5 months (range, 3 to 5 months) and 5.6 months (range, 4 to 12 months) after surgery in the experimental group and control group, respectively (Fig. 3), showing a significant difference (p = 0.023).
The main pathological features of AS is enthesitis and heterotopic ossification at the insertion of a tendon, ligament or joint capsule into bone causes ankylosis.10) The bone mass is maintained by the balance between the osteoblast and osteoclast activities, and an imbalance in the activities causes heterotopic ossification in AS.
The precise mechanisms of heterotopic ossification and ankylosis need to be established. Bone formation and remodeling are believed to be regulated by Wnt signaling, and the levels of Dickkopf-1 (DKK-1), a Wnt inhibitor, are low in AS patients.11,12) Wnt proteins, which are a family of 19 glycoproteins, have a major impact on osteoblastogenesis and bone growth in fetuses. Signal transduction from the cell-surface receptor to intracellular proteins promotes the differentiation of mesenchymal stem cells into osteoblast precursor cells and osteoblasts followed by bone formation.11-14) DKK-1 and Sfrp1 extracellular proteins are the major intracellular regulators that block Wnt signaling and there are many intracellular regulators. Among them, DKK-1 is believed to be the strongest inhibitor. Diarra et al.15) reported that the inhibition of DKK-1 led to bone formation in the peripheral joints of their animal subjects. Uderhardt et al.16) reported sacroiliac joint ankylosis following a blockade of DKK-1 in a rat model.
Bone morphogenetic proteins (BMP) play a major role in intraarticular heterotopic ossification and ankylosis.17,18) BMP is a member of the TGF-β superfamily, regulates cell proliferation, differentiation and lineage determination, and particularly promotes endochondral bone formation. The levels of BMP-2, BMP-6, and BMP-7 are high in AS patients with BMP-2 involved in early stages and BMP-6 and BMP-7 in the later stages.19)
Rapid bony union in AS patients might be due to the activation of the Wnt pathway and BMP, which promote bone remodeling through bone formation and bone induction.
A range of bone substitutes were used instead of autogenous grafts in the experimental group that are known to be the most effective materials for producing higher union rates than allogenous grafts. Nevertheless, bone union was observed in all AS patients and the interval to bone union was remarkably shorter than in the control group. Radiographs taken 3 months after surgery showed that grade 3 bone union had been achieved in 2, 3, and 4 levels in 7, 2, and 1 case, respectively in the experimental group, whereas it was obtained in 50% of the total levels at 5 months after surgery in the control group.
The limitations of this study are that the study population was small and the cause of early bone union in AS patients was not identified. Therefore, studies involving a large number of subjects focusing on the cause of early union are recommended. Despite these limitations, this study is the first to examine the grafted bone union in AS patients, and the clinical impression of early union in AS patients was confirmed by radiography.
The union of grafted bone was obtained earlier in AS patients treated with an osteotomy than in those with degenerative spinal diseases treated by decompression. Considering the early stage bone loss and high incidence of osteoporosis in AS patients, the early union of grafted bone after surgery for kyphosis is an important factor for determining the time to return to normal life and use a spinal brace.
Figures and Tables
Fig. 1
The result of grafted bone union according to Christensen's classification. AS: Ankylosing spondylitis, DSD: Degenerative spinal disease

Fig. 2
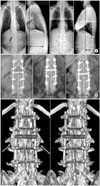
(A) Kyphotic deformity due to ankylosing spondylitis in a 31-year-old man. Correction was achieved after pedicle subtraction osteotomy at L3. (B) Lumbar spine radiographs were made 1, 2, and 3 months after osteotomy. Union of left side was observed. However, the right side was obscure at 3 months after the osteotomy. (C) Three-dimensional computed tomography scan demonstrate that both side grafted bone union was achieved 3 months after the osteotomy. POD: Postoperative day
References
1. Goel MK. Vertebral osteotomy for correction of fixed flexion deformity of the spine. J Bone Joint Surg Am. 1968. 50(2):287–294.

2. Kim KT, Lee SU, Kim YW, Kwon OS, Cho CH. Posterior closed wedge lumbar osteotomy in the kyphotic deformity of ankylosing spondylitis. J Korean Orthop Assoc. 1997. 32(7):1756–1765.

3. Urist MR. Osteotomy of the cervical spine: report of a case of ankylosing rheumatoid spondylitis. J Bone Joint Surg Am. 1958. 40(4):833–843.
4. Meenan RF, Gertman PM, Mason JH, Dunaif R. The arthritis impact measurement scales: further investigations of a health status measure. Arthritis Rheum. 1982. 25(9):1048–1053.

5. Herbert JJ. Vertebral osteotomy for kyphosis, especially in Marie-Strumpell arthritis: a report on fifty cases. J Bone Joint Surg Am. 1959. 41(2):291–302.
6. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985. (194):142–152.

7. Chang KW, Chen YY, Lin CC, Hsu HL, Pai KC. Closing wedge osteotomy versus opening wedge osteotomy in ankylosing spondylitis with thoracolumbar kyphotic deformity. Spine (Phila Pa 1976). 2005. 30(14):1584–1593.

8. Lenke LG, Bridwell KH, Bullis D, Betz RR, Baldus C, Schoenecker PL. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992. 5(4):433–442.

9. Christensen FB, Laursen M, Gelineck J, Eiskjaer SP, Thomsen K, Bunger CE. Interobserver and intraobserver agreement of radiograph interpretation with and without pedicle screw implants: the need for a detailed classification system in posterolateral spinal fusion. Spine (Phila Pa 1976). 2001. 26(5):538–543.

10. Zhang X, Aubin JE, Inman RD. Molecular and cellular biology of new bone formation: insights into the ankylosis of ankylosing spondylitis. Curr Opin Rheumatol. 2003. 15(4):387–393.

11. Polzer K, Diarra D, Zwerina J, Schett G. Inflammation and destruction of the joints: the Wnt pathway. Joint Bone Spine. 2008. 75(2):105–107.

12. Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med. 2007. 13(2):133–134.

13. Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006. 7(1-2):33–39.
14. Gregory CA, Gunn WG, Reyes E, et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci. 2005. 1049:97–106.

15. Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007. 13(2):156–163.

16. Uderhardt S, Diarra D, Katzenbeisser J, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010. 69(3):592–597.

17. Park MC, Park YB, Lee SK. Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 2008. 37(3):200–204.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download