Abstract
Rotator cuff deficient arthritis of the glenohumeral joint, especially cuff tear arthropathy, has proved a challenging clinical entity for orthopaedic surgeons ever since Charles Neer originally detailed the problem in 1983. Understanding has improved regarding the pathophysiology and pathomechanics underlying cuff tear arthropathy. Surgical reconstruction options can lead to excellent outcomes for patients afflicted with these painful and functionally limited shoulders. Humeral hemiarthroplasty and reverse total shoulder arthroplasty have jumped to the forefront in the treatment of cuff tear arthropathy. As studies continue to look at the results of these procedures in cuff tear arthropathy, existing indications and treatment algorithms will be further refined. In this article the history and pathophysiology of cuff tear arthropathy are reviewed. Additionally, the clinical findings and results of surgical reconstruction are discussed.
Cuff deficient arthritis of the glenohumeral joint encompasses a number of pathologies including rheumatoid arthritis or osteoarthritis without a competent rotator cuff, degenerative joint disease secondary to failed rotator cuff repair and cuff tear arthropathy (CTA). This article will focus on the history, pathophysiology, clinical manifestations and surgical treatment of CTA.
Robert Adams first described the clinical findings of CTA in 1857, however it was not until 1977 that Charles Neer coined the term "cuff tear arthropathy." Neer et al.1) went on to provide the first detailed description of CTA in 1983. CTA encompasses a condition characterized by a massive rotator cuff tear, proximal migration of the humerus resulting in femoralization of the humeral head and acetabularization of the acromion, glenoid erosion, loss of glenohumeral articular cartilage, osteoporosis of the humeral head and eventually humeral head collapse.1) In 1981 the entity referred to as "Milwaukee shoulder" was introduced by Halverson et al.2) Milwaukee shoulder involved hydroxyapatite crystals in the glenohumeral joint that lead to activated collagenase and protease activity, and thus joint destruction, in patients with rotator cuff deficiency. Neer considered the Milwaukee shoulder to be the same condition as CTA.
Based on his experience over an eight year period from 1975 to 1983, Neer et al.1) estimated that only 4% of patients with a complete tear of the rotator cuff go on to develop CTA. That such a small percentage of patients with complete tears of the rotator cuff end up developing CTA has led to debate over the years regarding the pathophysiology underlying CTA. Neer et al.1) believed that both nutritional and mechanical factors played a role in the development of CTA following a massive, chronic rotator cuff tear. Loss of the enclosed joint space leads to extravasation of synovial fluid, altered intra-articular pressure and impaired delivery of nutrients to the articular cartilage. Additionally, inactivity of the joint results in disuse osteoporosis and eventually collapse of the subchondral bone of the humeral head.1) The mechanical factors result from the loss of the dynamic stabilization and concavity compression normally provided by the rotator cuff. A massive rotator cuff tear can allow proximal humeral migration resulting in abnormal trauma and wear of the glenohumeral articular cartilage, glenoid, acromion, acromioclavicular joint and coracoid.1)
As previously mentioned, literature on the "Milwaukee shoulder" provides a hydroxyapatite crystal-mediated theory to explain the pathogenesis of CTA. This hypothesis posits that these crystals accumulate in glenohumeral joints with a massive rotator cuff tear. After being phagocytosed by cells in the synovium, collagenase and protease are released, causing destruction of articular cartilage and further soft tissue loss.2-4) This results in a positive feedback cycle where crystal-induced degradation leads to more crystal formation and thus, more tissue degradation. In 1997, Collins and Harryman5) synthesized Neer's theory on CTA pathogenesis with the crystal-mediated theory. Superior humeral migration that results from the loss of rotator cuff dynamic stability leads to abnormal trauma of the glenohumeral articular cartilage and the coracoacromial (CA) arch. This trauma releases particulate debris into the joint, setting off the previously described crystal-mediated inflammatory cascade.
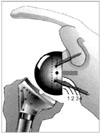
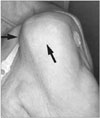
CTA tends to afflict the elderly, with women more likely to have the condition than men.6-9) Patients often present with complaints of chronic, progressive shoulder pain that is worse at night and with use of the shoulder.1,6-9) Additional complaints commonly include weakness and difficulty lifting the arm, leading to activity limitation.6,7) Physical examination reveals classic signs of a rotator cuff tear as well as atrophy of the supraspinatus and infraspinatus muscles.1,6-9) Shoulder swelling, termed the "fluid sign," can be seen from the escape of glenohumeral joint synovial fluid into the subacromial bursa (Fig. 1).1,7-9) Decreased active and passive range of motion, especially with elevation and external rotation, are typically appreciated.1,6-9) In some cases, the severely altered biomechanics can result in pseudoparalysis of the shoulder.
Radiographs of the shoulder comprise an important step in the diagnosis and evaluation of CTA. Glenohumeral arthritis, osteopenia of the humeral head and superior migration of the humeral head, along with its sequelae, can be seen. Humeral migration leads to changes in the acromion, acromioclavicular joint, coracoid and glenoid. Abnormal contact between the humerus and the acromion can lead to rounding off of the greater tuberosity (femoralization) and concave erosion of the underside of the acromion (acetabularization).10) Superior glenoid erosion is another common sequela of superior humeral head migration. Migration results in a decreased acromiohumeral interval (AHI) on anteroposterior (AP) radiographs, which is the distance from the undersurface of the acromion to the superior aspect of the humeral head. Saupe et al.11) showed that the size of rotator cuff tendon tears and the extent of fatty infiltration of the rotator cuff muscles have a significant negative correlation with the AHI (p < 0.05). Keener et al.12) similarly found that proximal humeral migration correlates significantly with the size of a rotator cuff tear. Hamada et al.13) used the AHI on AP radiographs as the basis for a radiographic classification system of massive rotator cuff tears. In grade 1 the AHI is greater than 6 mm and in grade 2 the AHI is 5 mm or less. Grade 3 adds acetabularization of the acromion to the criteria for grade 2. Grade 4 adds glenohumeral joint space narrowing to the criteria for grade 3. In grade 5, humeral head collapse is seen.13)
Other imaging modalities are not necessary to diagnose CTA but can provide additional information, assist in confirming the diagnosis, and aid in operative planning. Computed tomography (CT) can provide a more detailed view of the bony architecture that can be useful for determining the extent of bone erosion when planning treatment. Magnetic resonance imaging (MRI) provides detailed information on the soft tissue structures of the shoulder, most notably the extent of the rotator cuff tear and the quality of the rotator cuff muscles and tendons (Fig. 2).
Neer et al.1) wrote in their original 1983 paper on CTA that, "...surgical reconstruction of these shoulders is especially difficult." Since the rotator cuff plays a particularly important role in shoulder stabilization, loss of a functioning cuff has proved problematic when attempting to treat these shoulders surgically. Unconstrained total shoulder arthroplasty (TSA) was used by Neer et al. to treat 26 shoulders with CTA and resulted in poor functional outcomes, thus requiring an alternative "limited-goals" category to assess the outcome in these shoulders.1,14) These "limited-goals" were less than 20° of external rotation and 90° of elevation post-operatively.14) According to Neer's "limited-goals" category, an outcome was considered successful if the patient had no pain or mild pain, was pleased with the outcome of the procedure, and was capable of self-care. Using the "limited-goals" criteria, unconstrained total arthroplasty was considered successful in these 26 patients.1) The major problem with TSA in patients with CTA is loosening of the glenoid component, leading to the cessation of TSA as a treatment option for these patients. The proximal humeral head migration seen with a deficient rotator cuff leads to eccentric loading of the superior aspect of the glenoid component. Over time this eccentric loading leads to loosening of the glenoid component, an occurrence that Franklin et al termed the "rocking horse glenoid."15) Constrained and semiconstrained total shoulder prostheses were attempted with the idea that they would prevent proximal humeral migration and thus the eccentric loading of the superior aspect of the glenoid component. However, these prostheses actually resulted in increased stresses at the superior interface of the glenoid and the component and therefore high rates of glenoid component loosening.16,17)
Since the major problem with TSA in shoulders with a deficient rotator cuff was loosening of the glenoid component, humeral hemiarthroplasty (HHR), which avoids the glenoid component all together, was the next logical alternative to surgically reconstruct these shoulders. In 1992 Pollock et al.17) compared HHR to unconstrained TSA in 30 shoulders with glenohumeral arthritis and a deficient rotator cuff, with an average follow-up of 41 months. HHR was performed in 19 of the 30 shoulders and TSA in the other 11. Of note, 17 of the 30 shoulders were afflicted with inflammatory arthritis and 13 had CTA. Results showed equal pain relief for HHR and TSA. However, active forward elevation was significantly better in the HHR group (an average increase of 52° to a post-operative average of 112°) when compared to the TSA group (an average increase of 2° to a post-operative average of 82°). HHR also provided the benefits of a shorter and technically easier surgery, as well as an easier rotator cuff repair because of less humeral lateralization. As previously mentioned, HHR also avoids the problem of the "rocking horse glenoid." Results such as these led to the transition from TSA to HHR for shoulders with CTA, and spurred numerous studies on the use of HHR in CTA. Results from these studies showed no pain or mild pain in 47-86% of shoulders with glenohumeral arthritis and a deficient rotator cuff treated with HHR.18-21) Active forward elevation was found to increase by an average of 17° to 50°.18-21) Additionally, based on Neer's "limited-goals" criteria, between 63% and 86% of HHR's were considered to have successful outcomes.18,19,21)
Studies also began to delineate features in patients that could help predict the success of HHR for CTA. Sanchez-Sotelo et al.21) pointed out the importance of an intact coracoacromial (CA) arch in shoulders receiving a HHR. Shoulders without an intact CA arch are more likely to have progressive proximal humeral head migration, instability and worse post-operative gains in forward elevation. Goldberg et al.22) followed, for an average of 8.5 years, 40 shoulders treated with HHR for glenohumeral arthritis and a massive rotator cuff tear. They found that patients with a preoperative forward elevation of 90° or greater benefitted the most from HHR with regards to pain relief, function and ASES score.
Based on the results from the studies on HHR in CTA it became apparent that while HHR helped many patients and was certainly preferable to TSA, there were still a substantial number of patients left with painful and unsatisfactory shoulders. In 1985 Paul Grammont designed the Delta reverse total shoulder prosthesis.23) The main goal of the reverse prosthesis was to provide a fixed center of rotation allowing the deltoid to rotate the humerus even without an intact rotator cuff providing concavity compression. In reverse prostheses the concave component replaces the humeral head and the convex component is fixed to the glenoid. This results in a "humerosocket" and a "glenosphere." The new center of rotation for the humerus lies at the center of the glenosphere. This new center of rotation is medialized compared to normal glenohumeral anatomy, which lengthens the moment arm of the deltoid and allows for more recruitment of the anterior and posterior deltoid in abduction. Additionally, with the arm at the side the humerus is lowered relative to the acromion, which tensions the deltoid muscle fibers.23,24) All of these factors combine to allow the deltoid to elevate the humerus even though there is a deficient rotator cuff.
As acceptance and use of the reverse prosthesis has grown, a number of different designs have been introduced. There is the DePuy Delta based off Grammont's original design, the Tornier Aequalis, the Encore RSP, the Zimmer Anatomical Shoulder Inverse/Reverse, and the Zimmer TM Reverse. Studies on these modern reverse prostheses have consistently shown them to be effective at improving pain and functionality.23,25-29) Reverse prostheses have become particularly useful for the subset of patients in which a HHR has been shown to be less effective, notably the patients with no remaining rotator cuff, a violated CA arch, or less than 90° of forward elevation preoperatively. However, the big problem that has plagued the reverse prosthesis has been the high prevalence of complications, especially instability, infection and scapular notching. Walch et al.30) looked at the complications resulting from reverse total shoulder arthroplasty (RTSA) over a 10-year period at five surgical centers with a minimum of two years of follow-up. The overall postoperative complication rate in the 363 primary RTSA cases was 12.6% (revision RTSA has a higher complication rate). The most frequent post-operative complications encountered were instability (3.3%), humeral complications (2.8% - including fractures and component loosening and disassembly), glenoid complications (2.5% - component loosening and disassembly) and infection (2.2%). In the 149 cases of CTA in the study, the postoperative complication rate was 11.4% and the reoperation rate was 4%.30) Other studies on RTSA with at least 45 shoulders have reported reoperation and revision rates from 5% up to 33%.23,25-27,31)
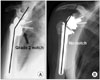
Among the complications of RTSA, scapular notching has garnered a great deal of attention, in part because of its high prevalence (reported as high as 96%)25) and also because of its still unclear relevance to clinical outcome. Scapular notching occurs when the humeral cup impinges on the scapular neck with the arm in adduction, causing erosion of the scapular neck.32) In 2008 Levigne et al.32) reported on a series of 337 shoulders treated with a RTSA (132 with CTA) with an average follow-up of 47 months. The overall prevalence of scapular notching was 62%, however it was found that shoulders treated for CTA had a significantly higher prevalence of notching at 76% (p = 0.0004). The classification system proposed by Sirveaux et al.27) is used to grade scapular notching (Fig. 3). Grade 1 notching involves only scapular bone. Grade 2 notching contacts the inferior screw of the baseplate. Grade 3 notching extends to the superior aspect of the inferior screw of the baseplate. Grade 4 notching extends superior to the inferior screw of the baseplate to include the area under the baseplate. In their series, Levigne et al.32) found that the prevalence of notching and the graded severity of the notching both increased over time. It is unclear though if scapular notching affects clinical outcome in RTSA. Levigne et al.32) found no association between notching and a lower constant score, decreased range of motion, pain or glenoid component loosening. However, Sirveaux et al.27) did find an association between grade 3 and 4 notching and a lower constant score (p < 0.05).
While the jury is still out on the significance of scapular notching, attempts have already been made to try and decrease the incidence of scapular notching. Wiater33) looked at the neck-shaft angle of the humeral component and the center of rotation offset of the glenoid component to determine their effects on notching. In 112 patients with inferiorly tilted glenospheres, 43 shoulders received a neck-shaft angle of 155° and no center of rotation offset (group A, Fig. 4A) while 69 shoulders received a neck-shaft angle of 143° and a 2.5 mm center of rotation offset (group B, Fig. 4B). Group A was 10 times more likely to have notching (33 of 43 shoulders) than group B (4 of 69 shoulders). Connor reported on a prospective series of 112 RTSA's with patients receiving either a humeral component with a neck-shaft angle of 155° or a humeral component with a neck-shaft angle of 143°. The patients receiving the 143° design had higher postoperative ASES scores and a significantly lower rate of notching (22.7% vs. 59.5%, p < 0.01). Furthermore, 28% of the shoulders with the 155° design had grade 2 or higher notching while no shoulders with the 143° design had notching beyond grade 1.34)
Concerns have also arisen regarding the durability of reverse prostheses. Guery et al.31) focused on this question when they looked at the long-term survivorship of reverse prostheses in 60 shoulders. With endpoints of implant replacement, glenoid loosening, and a constant score of < 30, survivorship at 10 years was found to be 91%, 84%, and 58% respectively. Additionally, survivorship with regards to replacement was significantly better in shoulders that received RTSA for CTA when compared to shoulders that received RTSA for a different disorder (95% vs. 77%, p < 0.01).31) Sirveaux et al.27) found similar results in their study of 77 shoulders that received a reverse prosthesis. At five years, 91.3% had survived without component loosening or revision.
While CTA remains a difficult clinical entity, much progress has been made since Neer's seminal paper on the topic in 1983. Knowledge about the clinical diagnosis, imaging features and indicators of severity has improved the ability to recognize CTA. Progress on delineating the pathophysiology and pathomechanics involved in CTA has shed light on the disease, although more work is needed. And lastly, the surgical reconstruction of CTA has evolved to a great extent. Outcomes with the original use of unconstrained and constrained TSA were unacceptable and thus TSA gave way to newer approaches to treat CTA such as HHR and RTSA. HHR has become the treatment of choice in younger, active patients with an active forward elevation > 90°, an intact CA arch, minimal superior humeral migration and some torn rotator cuff that is amenable to repair. RTSA has emerged as the treatment of choice in older, more sedentary patients with an active forward elevation < 90°, superior humeral migration, no rotator cuff amenable to repair, good glenoid bone and an intact deltoid. As more data is gathered on the treatment of CTA, especially on the role, viability and survivorship of reverse prostheses, these indications for specific treatments will be further refined.
Figures and Tables
 | Fig. 2(A) Coronal oblique MRI showing a massive rotator cuff tear. (B) Sagittal MRI showing fatty infiltration and atrophy of the supraspinatus muscle. |
 | Fig. 3Sirveaux classification for scapular notching (reproduced from Sirveaux F, et al. J Bone Joint Surg Br. 2004;86(3):388-95, with permission from British Society of Bone and Joint Surgery).27)
|
References
1. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983. 65(9):1232–1244.

2. Halverson PB, Cheung HS, McCarty DJ, Garancis J, Mandel N. "Milwaukee shoulder": association of microspheroids containing hydroxyapatite crystals, active collagenase, and neutral protease with rotator cuff defects. II. Synovial fluid studies. Arthritis Rheum. 1981. 24(3):474–483.

3. McCarty DJ, Halverson PB, Carrera GF, Brewer BJ, Kozin F. "Milwaukee shoulder": association of microspheroids containing hydroxyapatite crystals, active collagenase, and neutral protease with rotator cuff defects. I. Clinical aspects. Arthritis Rheum. 1981. 24(3):464–473.

4. Garancis JC, Cheung HS, Halverson PB, McCarty DJ. "Milwaukee shoulder": association of microspheroids containing hydroxyapatite crystals, active collagenase, ad neutral protease with rotator cuff defects. III. Morphologic and biochemical studies of an excised synovium showing chondromatosis. Arthritis Rheum. 1981. 24(3):484–491.

5. Collins DN, Harryman DT 2nd. Arthroplasty for arthritis and rotator cuff deficiency. Orthop Clin North Am. 1997. 28(2):225–239.

6. Ecklund KJ, Lee TQ, Tibone J, Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007. 15(6):340–349.

7. Feeley BT, Gallo RA, Craig EV. Cuff tear arthropathy: current trends in diagnosis and surgical management. J Shoulder Elbow Surg. 2009. 18(3):484–494.

8. Jensen KL, Williams GR Jr, Russell IJ, Rockwood CA Jr. Rotator cuff tear arthropathy. J Bone Joint Surg Am. 1999. 81(9):1312–1324.
9. Zeman CA, Arcand MA, Cantrell JS, Skedros JG, Burkhead WZ Jr. The rotator cuff-deficient arthritic shoulder: diagnosis and surgical management. J Am Acad Orthop Surg. 1998. 6(6):337–348.

10. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004. 86:Suppl 2. 35–40.
11. Saupe N, Pfirrmann CW, Schmid MR, Jost B, Werner CM, Zanetti M. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006. 187(2):376–382.

12. Keener JD, Wei AS, Kim HM, Steger-May K, Yamaguchi K. Proximal humeral migration in shoulders with symptomatic and asymptomatic rotator cuff tears. J Bone Joint Surg Am. 2009. 91(6):1405–1413.

13. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: a long-term observation. Clin Orthop Relat Res. 1990. (254):92–96.

14. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982. 64(3):319–337.

15. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty: association with rotator cuff deficiency. J Arthroplasty. 1988. 3(1):39–46.
16. Orr TE, Carter DR, Schurman DJ. Stress analyses of glenoid component designs. Clin Orthop Relat Res. 1988. (232):217–224.

17. Pollock RG, Deliz ED, McIlveen SJ, Flatow EL, Bigliani LU. Prosthetic replacement in rotator cuff-deficient shoulders. J Shoulder Elbow Surg. 1992. 1(4):173–186.

18. Field LD, Dines DM, Zabinski SJ, Warren RF. Hemiarthroplasty of the shoulder for rotator cuff arthropathy. J Shoulder Elbow Surg. 1997. 6(1):18–23.

19. Williams GR Jr, Rockwood CA Jr. Hemiarthroplasty in rotator cuff-deficient shoulders. J Shoulder Elbow Surg. 1996. 5(5):362–367.

20. Zuckerman JD, Scott AJ, Gallagher MA. Hemiarthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2000. 9(3):169–172.

21. Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001. 83(12):1814–1822.

22. Goldberg SS, Bell JE, Kim HJ, Bak SF, Levine WN, Bigliani LU. Hemiarthroplasty for the rotator cuff-deficient shoulder. J Bone Joint Surg Am. 2008. 90(3):554–559.

23. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005. 14:1 Suppl S. 147S–161S.
24. Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009. 17(5):284–295.
25. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005. 87(7):1476–1486.
26. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005. 87(8):1697–1705.
27. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004. 86(3):388–395.
28. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008. 90(6):1244–1251.

29. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002. 25(2):129–133.

30. Walch G, Wall B, Mottier F. Walch G, Boileau P, Mole D, Favard L, Levigne C, Sirveaux F, editors. Complications and revision of the reverse prosthesis: a multicenter study of 457 cases. Reverse shoulder arthroplasty: clinical results, complications, revision. 2006. Montepellier: Sauramps Medical;335–352.
31. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006. 88(8):1742–1747.
32. Levigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008. 17(6):925–935.

33.
LU Bigliani
Columbia University Medical Center
. Conversation with: Wiater M (Beaumont Hospital, Royal Oak, MI). 2010. 07. 20.
34.
LU Bigliani
Columbia University Medical Center
. Conversation with: Connor P (OrthoCarolina, Charlotte, NC). 2010. 07. 20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download