Abstract
Purpose
This study was aimed to evaluate the effect of the Freeze Dried Bone Allograft and Demineralized Bone Matrix on osseous regeneration in the rat calvarial defects.
Methods
Eight mm critical-sized calvarial defects were created in the 80 male Sprague-Dawley rats. The animals were divided into 4 groups of 20 animals each. The defects were treated with Freeze Dried Bone Allograft(SureOss™), Demineralized Bone Matrix(ExFuse™ Gel, ExFuse™ Putty), or were left untreated for sham-surgery control and were evaluated by histologic and histomorphometric parameters following a 2 and 8 week healing intervals. Statistical analysis was done between each groups and time intervals with ANOVA and paired t-test.
Results
Defect closure, New bone area, Augmented area in the SureOss™, ExFuse™ Gel, ExFuse™ Putty groups were significantly greater than in the sham-surgery control group at each healing interval(P < 0.05). In the New bone area and Defect closure, there were no significant difference between experimental groups. Augmented area in the ExFuse™ Gel, ExFuse™ Putty groups were significantly greater than SureOss™ group at 2weeks(P < 0.05), however there was no significant difference at 8 weeks.
Figures and Tables
 | Figure 2Representative photomicro graph of defect site receiving the sham-surgery at 2 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
 | Figure 3Representative photomicro graph of defect site receiving the sham-surgery at 2 weeks (defect margin, PB : pre-existing bone, NB : new bone ; HE stain, magnification × 100). |
 | Figure 4Representative photomicro graph of SureOss™ at 2 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
 | Figure 5Representative photomicro graph of SureOss™ at 2 weeks (defect margin, PB : pre-existing bone, NB : new bone ; HE stain, magnification × 100). |
 | Figure 6Representative photomicro graph of ExFuse™ Gel at 2 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
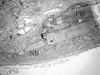 | Figure 7Representative photomicro graph of ExFuse™ Gel at 2 weeks (defect margin, PB : pre-existing bone, NB : new bone ; HE stain, magnification × 100). |
 | Figure 8Representative photomicro graph of ExFuse™ Putty at 2 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
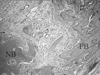 | Figure 9Representative photomicro graph of ExFuse™ Putty at 2 weeks (defect margin, PB : pre-existing bone, NB : new bone ; HE stain, magnification × 100). |
 | Figure 10Representative photomicro graph of defect site receiving the sham-surgery at 8 weeks (arrow heads : (defect margin ; HE stain, magnification × 20). |
 | Figure 11Representative photomicro graph of defect site receiving the sham-surgery at 8 weeks (defect margin, PB : pre-existing bone, NB : new bone ; HE stain, magnification × 100). |
 | Figure 12Representative photomicro graph of SureOss™ at 8 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
 | Figure 13Representative photomicro graph of SureOss™ at 8 weeks (defect margin, NB : new bone ; HE stain, magnification × 100). |
 | Figure 14Representative photomicro graph of ExFuse™ Gel at 8 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
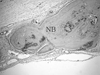 | Figure 15Representative photomicro graph of ExFuse™ Gel at 8 weeks (defect margin, NB : new bone ; HE stain, magnification × 100). |
 | Figure 16Representative photomicro graph of ExFuse™ Putty at 8 weeks (arrow heads : defect margin ; HE stain, magnification × 20). |
 | Figure 17Representative photomicro graph of ExFuse™ Putty at 8 weeks (defect margin, NB : new bone ; HE stain, magnification × 100). |
References
2. Lane JM. Bone graft substitutes. West J Med. 1995. 163:565–566.
3. Hislop WS, Finlay PM, Moos KF. A preliminary study into the uses of anorganic bone in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 1993. 31:149–153.

4. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989. 60:655–663.

5. Kim Sung-Hee, Kim Chong Kwan, Chai Jung-kiu, Cho Kyoo-Sung. Histologic Study on Healing after Implantation of several Bone Substitutes in Rat Calvarial Defects. J Korean Acad Periodontol. 1994. 24:618–632.

6. Lee EJ, Chung HJ. Histologic Study on Healing after Implantation of several Bone Substitutes in Rat Calvarial Defects. J Korean Acad Periodontol. 1998. 28:87–102.

7. Schepers EJ, Ducheyne P, Barbier L, Schepers S. Bioactive glass particles of narrow size range: a new material for the repair of bone defects. Implant Dent. 1993. 2:151–156.
8. Urist MR, States BS. Bone formation by augmentation. Science. 1965. 150:892.
9. Libin BM, Ward HL, Fishman L. Decalcified, lyophilized bone allografts for use in human periodontal defects. J Periodontol. 1975. 46:51–56.

10. Acil Y, Springer IN, Broek V, Terheyden H, Jepsen S. Effects of bone morphogenetic protein-7 stimulation on osteoblasts cultured on different biomaterials. J Cell Biochem. 2002. 86:90–98.

11. Wikesjo UM, Sorensen RG, Kinoshita A, Wozney JM. RhBMP-2/alphaBSM induces significant vertical alveolar ridge augmentation and dental implant osseointegration. Clin Implant Dent Relat Res. 2002. 4:174–182.
12. Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996. 17:1127–1131.

13. Bowers GM, Chadroff B, Carnevale R, et al. Histologic evaluation of new attachment apparatus formation in humans. Part I. J Periodontol. 1989. 60:664–674.
14. Bowers GM, Chadroff B, Carnevale R, et al. Histologic valuation of new attachment apparatus formation in humans. Part II. J Periodontol. 1989. 60:675–682.
15. Strates BS, Tiedeman JT. Contribution of osteoinductive and osteoconductive properties of demineralized bone matrix to skeletal repair. Eur J Exp Musculoskel Res. 1993. 2:61.
16. Chesmel Kathleen D, Branger Judith, Wertheim Heiman, Scarborough Nelson. Healing response to various forms of humans demineralized bone matrix in athymic rat cranial defects. J Oral Maxillofac Surg. 1998. 56:857–863.

17. Bowers GM, Chadroff B, Carnevale R, et al. Histologic evaluation of new attachment apparatus formation in humans. Part III. J Periodontol. 1989. 60:683–693.
18. Hong So Mi, Herr Yeek, Kwon Young Hyuk, Park Joon Bong. Space-maintaining and osteopromotive effect of freeze-dried bone graft in the procedure of GBR. J Korean Acad Periodontol. 2004. 34:149–162.

19. Garg Arun K. Bone biology, harvesting, and grafting for dental implants : rationale and clinical applications. 2004. Quintessence;21–57.
20. Mellonig JT, Bowers GM, Cotton WR. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: a histological evaluation. J Periodontol. 1981. 52:297–302.

21. Mellonig JT, Bowers GM, Bright RW, Lawrence JJ. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J Periodontol. 1976. 47:125–131.

22. Sepe WW, Bowers GM, Lawrence JJ, Friedlaender GE, Koch RW. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects-part II. J Periodontol. 1978. 49:9–14.

23. Frame JW. Hydroxyapatite as a biomaterial for alveolar ridge augmentation. Int J Oral Maxillofac Surg. 1987. 16:642–655.

24. Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by combined hydroxylapatite and osteoinductive material. Scand J Dent Res. 1991. 99:64–74.

25. Babbush CA. Histologic evaluation of human biopsies after dental augmentation with a demineralized bone matrix putty. Implant Dent. 2003. 12:325–332.

26. Matzenbacher SA, Mailhot JM, McPherson JC 3rd, et al. In vivo effectiveness of a glycerol-compounded demineralized freeze-dried bone xenograft in the rat calvarium. J Periodontol. 2003. 74:1641–1646.

27. Senn N. On the Healing of Aseptic Bone Cavities by Implantation of Antiseptic Decalcified Bone. Am J Med Sci. 1889. 10:352–368.

28. Mellonig JT, Bowers GM, Bright RW, Lawrence JJ. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J Periodontol. 1976. 47:125–131.

29. Meffert RM. Current usage of bone fill as an adjunct in implant dentistry. Dent Implantol Update. 1998. 9:9–12.
30. Herold RW, Pashley DH, Cuenin MF, et al. The effects of varying degrees of allograft decalcification on cultured porcine osteoclast cells. J Periodontol. 2002. 73:213–219.

31. Yukna RA, Vastardis S. Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol. 2005. 76:57–65.

32. Rosen PS, Reynolds MA. A retrospective case series comparing the use of demineralized freeze-dried bone allograft and freeze-dried bone allograft combined with enamel matrix derivative for the treatment of advanced osseous lesions. J Periodontol. 2002. 73:942–949.

33. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989. 60:655–663.

34. Cammack GV 2nd, Nevins M, Clem DS 3rd, Hatch JP, Mellonig JT. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005. 25:231–237.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download