Abstract
Purpose
The purpose of this study was to evaluate the bone regeneration of particulated hydroxyapatite(HA) and block type of hydroxyapatite graft in rabbit calvarial defects.
Methods
An 8 mm calvarial circular defects were created in sixteen young adult New Zealand white male rabbits (weight 3.0~3.5 kg). Each defects were filled with Bio-Oss, particulated HA and block type HA. Sham surgery control defects were filled with blood clots. The specimens were harvested at 4 weeks and 8 weeks for histologic and histomorphometric evaluation.
Results
Histomorphometric analysis demonstrated statistical differences in defect closure, new bone formation, and bone density of the four groups. Block type of HA group showed increased bone formation and bone density at 4 weeks and 8 weeks compared with Bio-Oss group or sham surgery control group(p<0.05).
Figures and Tables
Figure 7
Higher magnification of Bio-Oss group presenting new bone (NB) with Bio-Oss remnants (arrow). (×100, H-E).
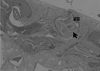
Figure 8
Histologic section of particulated HA group at 4 weeks (×10, H-E). Arrow head: defect margin.

Figure 9
Histologic section of particulated HA group at 8 weeks (×10, H-E). Arrow head: defect margin.

Figure 10
Higher magnification of particulated HA group presenting new bone (NB) with HA remnants (arrow). (×100, H-E).
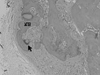
Figure 11
Histologic section of block type HA group at 4 weeks (×10, H-E). Arrow head: defect margin.

Figure 12
Histologic section of block type HA group at 8 weeks (×10, H-E). Arrow head: defect margin.

Figure 13
Higher magnification of block type HA group presenting new bone (NB) with osteoblastic steam (arrow). (×100, H-E).
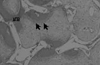
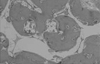
Figure 14
Higher magnification of block type HA group showing new bone with numerous blood vessel (×100, H-E).
References
1. Hiatt WH, Schallhorn RG. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J Periodontol. 1973. 44:194–208.

2. Mellonig JT. Periodontal bone graft technique. Int J Periodontics Restorative Dent. 1990. 10:288–299.
4. Pinholt EM, Solheim E, Bang G, Sudmann E. Bone induction by composites of bioresorbable carriers and demineralized bone in rats: a comparative study of fibrin-collagen paste, fibrin sealant, and polyorthoester with gentamicin. J Oral Maxillofac Surg. 1992. 50:1300–1304.

5. Schwartz Z, Somer A, Mellonig JT, Carnes DL Jr, Dean DD, Cochran DL, Boyan BD. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on age but not gender. J Periodontol. 1998. 69:470–478.

6. Somerman MJ. Is there a role for DFDBA in periodontal regenerative therapy? J Periodontol. 1996. 67:946–948.
7. Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000. 11:217–229.

8. Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by Bio-Oss. Scand J Dent Res. 1991. 99:154–161.

9. Haas R, Mailath G, Dortbudak O, Watzek G. Bovine hydroxyapatite for maxillary sinus augmentation: Analysis of interfacial bond strength of dental implants using pull-out tests. Clin Oral Implants Res. 1998. 9:117–122.
10. Artzi Z, Nemcovsky CE, Dayan D. Bovine-HA spongiosa blocks and immediate implant placement in sinus augmentation procedures. Histopathological and histomorphometric observations on different histological stainings in 10 consecutive patients. Clin Oral Implants Res. 2002. 13:420–427.

11. Carmagnola D, Berglundh T, Lindhe J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. A experimental study in Labrador dogs. J Clin Periodontol. 2002. 29:377–383.

12. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003. 8:227–265.

13. Carranza FA Jr, Kennedy EB, Lekovic V, Taltmante E, Valencia J, Dimitrijevic B. Histologic study of the healing of human periodontal defect after placement of porous hydroxyapatite implants. J Periodontol. 1987. 58:682–688.

14. Stahl SS, Froum SJ. Histologic evaluation of human intraosseous healing response to the placement of tricalcium phosphate ceramic implant. J Periodontol. 1986. 57:211–217.

15. de Bruijn JD, van Blitterswijk CA, Davies JE. Initial bone matrix formation at the hydroxyapatite interface in vivo. J Biomed Mater Res. 1995. 29:89–99.
16. Cerroni L, Filocamo R, Fabbri M, Piconi C, Caropresso S, Condo SG. Growth of osteoblast like cells on porous htdroxyapatite ceramics: an in vitro study. Biomol Eng. 2002. 19:119–124.

17. Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999. 20:2287–2303.

18. Moskow BS, Bubarr A. Histological assessment of human periodontal defects after durapatite ceramic implants. J Periodontol. 1983. 54:455–462.

19. Yukna RA, Harrison BG, Caudill RF. Evaluation of durapatite ceramic as an alloplastic implant in periodontal osseous defects. Twelve month reentry results. J Periodontol. 1985. 56:540–547.

20. Choi Jung-Yoo, Chae Gyung-Joon, Kim Chang-Sung, et al. The effects of novel biodegradable amorphorous calcium phosphate on bone regeneration in rat calvarial defects. J Korean Acad Periodontol. 2007. 37:871–879.

21. Monroe EA, Votava W, Bass DB. New calcium phosphate ceramic material for bone and tooth implants. J Dent Res. 1971. 50:860–861.

22. Um Yoo-Jung, Hong Ji-Yeon, Kim Sung-Tae, et al. Bone formation of newly developed biphasic calcium phosphate in rabbit calvarial defect model: pilot study. J Korean Acad Periodontol. 2008. 38:163–170.

23. Lee Kwang-Ho, Jang Hyun-Seon, Park Joo-Cheol, et al. Bone formation effects of HA/β-TCP composite powder in rabbit calvarial bone defects: Histologic study. J Korean Acad Periodontol. 2006. 36:1–14.

24. Fabbi M, Celotti GC, Ravaglioli A. Hydroxyapatite-based porous aggregates -physicochemical nature, structure, texture and architecture. Biomaterials. 1995. 16:225–228.

25. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998. 19:133–139.

26. Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell substatum controls BMP-induced osteogenesis. J Biochem. 1997. 121:317–324.

27. Scarano A, Pecora G, Piattelli M. Osseointegration in a sinus augmented with bovine porous bone mineral: histological results in an implant retrieved 4 years after insertion. A case report. J Periodontol. 2004. 75:1161–1166.

28. Zaffe D, Leghissa GC, Pradelli J, Botticelli AR. Histological study on sinus lift grafting by Fisiograft and Bio-Oss. J Mater Sci Mater Med. 2005. 16:789–793.

29. Yildrim M, Spiekermann H, Handt A, Edelhoff D. Maxillary sinus augmentation with the xenograft Bio-Oss and autogenous intraoral bone for qualitative improvement of the implant site: a histological and histomorphometric clinical study in humans. Int J Oral Maxillofac Implants. 2001. 16:23–33.




 PDF
PDF Citation
Citation Print
Print











 XML Download
XML Download