Abstract
Purpose
Cytolethal distending toxin (CDT) is a family of heat-labile cytotoxins produced by several gram-negative mucosa-associated pathogens, including Aggregatibacter actinomycetemcomitans. CDT is well known to be capable of inducing growth arrest, morphological alterations, and eventually death in various cells. CDT belongs to a tripartite AB2 toxin (CdtB: the enzymatic A subunit; CdtA and CdtC: the heterodimeric B subunit). Previous studies proposed that CdtA and CdtC together bind to a cell surface receptor and glycolipids act as a receptor for A. actinomycetemcomitans CDT (AaCDT). In this study, recombinant CdtA and CdtC proteins of AaCDT were co-expressed in a bacterial expression system and tested for their affinity for GM1 ganglioside.
Methods
The genes for CdtA and CdtC from A. actinomycetemcomitans Y4 were utilized to construct the expression vectors, pRSET-cdtA and pET28a-cdtC. Both CdtA and CdtC proteins were expressed in Escherichia coli BL21(DE3) and then purified using hexahistidine (His6) tag. The identity of purified protein was confirmed by anti-His6 antibody and monoclonal anti-CdtA antibody. Furthermore, the affinity of recombinant protein to GM1 ganglioside was checked through ELISA.
Periodontitis is an inflammatory disease in the supporting tissues of the teeth caused by a composite of microorganisms. Aggregatibacter actinomycetemcomitanshas been suspected to be one of the key pathogens in the etiology of human periodontitis.
A. actinomycetemcomitans is a gram-negative coccobacillus has been implicated not only in the pathogenesis of localized aggressive periodontitis but also in several systemic diseases, such as endocarditis, meningitis, and osteomylitis. This organism is able to produce a variety of virulence factors that are involved in the colonization of the oral cavity, the destruction and inhibition of regeneration of the periodontal tissues, and interference with host defense mechansims. These virulence factors include collagenases, endotoxin, leukotoxin, and cytolethal distending toxin (CDT)1).
CDTs are a family of heat-labile proteinous cytotoxins produced by several gram-negative pathogens associated with mucosal surfaces, including Escherichia coli, Campylobacter jejuni, Shigella species, Haemophilus ducreyi, and A. actinomycetemcomitans, with the capacity to induce growth arrest, morphological alterations, and eventually death in the affected cells2).
CDT is found and expressed in the majority of the A. actinomycetemcomitans isolates. Leung et al.3) found that A. actinomycetemcomitanswas isolated from 67% of aggressive periodontal diseased subjects and all but one contained all three cdt genes. Ahmed et al.4) reported that 43 of 50 strains of A. actinomycetemcomitans isolated from periodontitis patients possessed all three cdt genes and expressed a cytotoxic activity. Also, Fabris et al.5) found that 34 of 40 A. actinomycetemcomitans detected all three cdt genes and 39 samples caused distension of Chinese hamster ovary cells.
The periodontium is composed of epithelial cells, fibroblasts, cememtoblasts, and osteoblasts. These various cell types respond differently to the CDT of A. actinomycetemcomitans. Belibasakis et al.6) reported CDT of A. actinomycetemcomitans inhibits the proliferation of gingival fibroblasts and periodontal ligament cells without affecting their viability, and the cell cycle arrest associated with this inhibition is a combined G1 and G2/M phase arrest. In contrast, Kanno et al.7) suggested that human periodontal ligament fibroblasts are resistant to the cytotoxic effects of the A. actinomycetemcomitans CDT.
Proliferation of human epithelial cell lines was rapidly inhibited by CDT7,8). By inducing growth arrest in oral epithelial cells, the toxin may interfere with the normal periodontal connective tissue remodeling equilibrium6), or may facilitate the invasion and multiplication of A. actinomycetemcomitans in the deeper periodontal tissue8), respectively.
A. actinomycetemcomitans up-regulates receptor activator of NF-kB ligand (RANFL) expression in gingival fibroblasts and periodontal ligament cells, and that CDT is its component primarily responsible for this event9). CDT can also stimulate RANKL expression in T-cells10). RANKL expression activates osteoclasts involved in bone resorption. RANKL expression in the periodontium has been implicated in physiological tooth eruption and orthodontic tooth movement, as well as in the pathological process of periodontitis11).
CDT toxin is encoded by three different genes: cdtA, cdtB, and cdtC, and expression of all three cdt genes is required in order to produce active CDT12). CDT is suggested to be a unique tripartite AB2 toxin in which CdtB is the active A subunit, and CdtA and CdtC constitute the heterodimeric B subunit2,13,14). Furthermore, it is generally agreed that the active subunit, CdtB, enters the cell while CdtA and CdtC remain associated with the cell surface14). The CdtB protein has been shown to possess DNase activity13) when introduced into or expressed within eukaryotic cells, and there seems to be a reasonable consensus that a functional CdtB molecule is essential for expression of toxicity by CDT2). The cellular responses to DNA damage lead to a characteristic G2/M cell cycle arrest, cellular distension, nuclear enlargement observed in intoxicated cells15).
While progress in understanding the function of CdtB has been made, there is still little known about the precise roles of CdtA and CdtC in CDT function. Mao and DiRienzo8) demonstrated by immunofluorescence assay that only CdtA gene derived from A. actinomycetemcomitans binds to the surfaces of Chinese hamster ovary cells independently. A study by Lee et al.16) with C. jejuni showed both CdtA and CdtC were able to bind specifically and independently to the surface of HeLa cells. But, many studies find that CdtA and CdtC together bind to the cellular membrane14,17,18). In the crystal structures of the A. actinomycetemcomitans holotoxins, CdtA and CdtC form ricin-like lectin domains that may play a role in recognizing the cellular receptor that delivers the CdtB subunit into the target cells14,19). The cell surface receptor for the CDT has not yet been identified. However, there is evidence that E. coli CdtA-II and CdtC-II bind N-linked fucose-containing complex carbohydrates on the surfaces of HeLa cells17). Other studies reported that glycosphingolipid GM1 and GM3 may play an important role as the receptor of A. actinomycetemcomitans CDT18). In addition, cholesterol-rich cytoplasmic membrane lipid rafts are also involved in the delivery of CdtB into target cell20).
The goal of this study was to co-express CdtA and CdtC subunits of CDT from A. actinomycetemcomitans and to find whether purified CdtA-CdtC protein binds to GM1 ganglioside.
A. actinomycetemcomitans Y4 (ATCC 43718) was cultured in Brain Heart Infusion broth medium for 24 hat 37℃ in anaerobic chamber containing 85% N2, 10% H2, and 5% CO221). Growth was monitored by optical density (OD) at 600 nm, with all batch cultures having an initial OD600 of approximately 0.01 after sub-culturing from overnight growth. For the co-expression of CdtA and CdtC subunit, E. coli BL21(DE3) transformed with pRSET-cdtA22) and pET28a-cdtC23) was cultured in Luria-Bertari (LB) medium at 37℃ with shaking at 200 rpm.
E. coli BL21(DE3) transformed with the pRSET-cdtA and pET28a-cdtC plasmid was cultured into 5 ml of LB broth containing 50 ug/ml for each of ampicillin and kanamycine. At the following day, the bacteria were freshly inoculated into the 500 ml LB broth containing 50 ug/ml ampicillin/kanamycine and further grown at 37℃ with vigorously shaking until OD600 reached to 0.8 and then induced by 1 mM of isopropyl-β-D-thiogalactopyranoside (IPTG) for another 3 h at 30℃.
Induced bacteria were harvested by centrifugation, and the resulting pellet was resuspended in binding buffer (20 mM Tris-HC l pH 8.0, 0.5 M NaCl, and 5 mM imidazole). The resuspended bacteria were disrupted with an ultrasonic disruptor24). The sonicated samples were centrifuged at 13000 rpm for 10 min and then the supernatant was saved as the soluble fractions. The soluble fractions were then loaded into the nickel-chelated agarose (Ni-NTA, Qiagen). The column was washed with binding buffer and eluted with binding buffer containing 200 mM imidazole.
The fractions containing recombinant protein (His6-tagged protein) were dialyzed and then resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Recombinant CdtA-CdtC protein were mixed with 100 µl of gel loading buffer and heated in a boiling water bath for 5 min. The samples were applied to 10-20% polyacrylamide gel. After electrophoresis, protein bands were transferred to a nitrocellulose membrane. The membrane was blocked with 3% BSA in TBS containing 0.1% Tween-20 (TBST) buffer for 1h at room temperature with shaking. The blocked membrane was incubated with anti-His6 antibody or monoclonal anti-CdtA antibody tagged with AP (alkaline phosphatase) and then washed in TBST. The membrane was treated with BCIP/NBT to induce developing reaction and incubated in the dark. The membrane was rinsed with H2O to stop the developing reaction.
GM1 ganglioside was used to coat Enzyme-Linked ImmunoSorbent Assay (ELISA) plate at 37℃ for 2 h and the coated plates was blocked by 1% skim milk. After washed using PBS (pH 7.5), the plates were incubated with purified CdtA-CdtC protein or CdtA in PBS at 37℃ for 2 h. Then the plates were washed using PBS and incubated with anti-His6 antibody or monoclonal anti-CdtA antibody in PBS at 37℃ for 2 h. The plates were then washed with PBS, and further incubated with anti-mouse IgG conjugated with alkaline phosphatase in PBS (1:5000 dilution) at 37℃ for 2 h and then applied with the substrate which is converted by the enzyme to elicit a chromogenic signal. The result was quantified using a spectrophotometer for OD at 405 nm.
CdtA-CdtC protein was expressed in E. coli BL21(DE3) transformd with pRSET-cdtA and pET28a-cdtC, and then purified by Ni-NTA affinity column. Recombinant CdtA and CdtC proteins were clearly shown in the expected locations of SDS-PAGE gel (Fig. 1).
Both CdtA and CdtC proteins were expressed as His6-tagged fusion proteins which were confirmed by anti-His6 antibody by Western blot. CdtA was shown just below 25 kDa and CdtC around 20 kDa (Fig. 2).
Monoclonal anti-CdtA antibody also only detected CdtA at 25kDa (Fig. 3). A protein at a higher band around 35 kDa was also purified by Ni-NTA column and responded to anti-His6 and anti-CdtA antibodies.
By using ELISA, purified C dtA-C dtC protein w as evaluated whether it bound to GM1 ganglioside. The peak OD405 value of purified CdtA-CdtC protein was measured a round 0.5. CdtA showed to have lower OD405 value than CdtA-CdtC protein at any dilution (Fig. 4).
Monoclonal anti-CdtA antibody was also applied to check GM1 affinity of purified CdtA-CdtC protein and CdtA. Recombinant CdtA-CdtC protein showed a higher OD405 value than recombinant CdtA protein (Fig. 5).
The crystal structures of the CDT cytotoxin from Aggregatibacter actinomycetemcomitansinclude a pronounced groove between the CdtA and CdtC subunits in the heterotoxin complex19). A CDT groove mutant exhibited diminished binding to cells25). This data support the hypothesis that the groove formed between CdtA and CdtC are involved in the binding of the toxin to target cells. Also, many studies reported that CdtA and CdtC together bind t o the cellular membrane14,17,18).
In this study, CdtA and CdtC subunits were expressed as soluble proteins from E. coli transformed with both cdtA- and cdtC-expressing vectors, while each of CDT subunit expressed a s an insoluble protein. These expressed CDT subunits were purified with Ni-NTA column and also responded to anti-His6 and anti-CdtA antibodies (Fig. 1-3). CdtA protein was located at two different bands in SDS-PAGE and Western blot (Fig. 1-3) and some studies also report similar results26,27). They reported that it was caused by a covalent bond between cystein residue of CdtA N-terminus and lipid27). Therefore, both of doublet bands in Fig. 1-3 are supposed to contain CdtA subunit.
The cell surface receptor for CDT has not been clearly identified. However, it has been reported that both CdtA and CdtC recognize N-linked fucose-containing glycoproteins17) or gangliosides, such as GM1 and GM318), in vitro. Therefore purified CdtA-CdtC protein was evaluated whether it bound to GM1 ganglioside through ELISA. Recombinant CdtA-CdtC protein had higher affinity to GM1 ganglioside than CdtA (Fig. 4 and 5). Either anti-His6 antibody or monoclonal anti-CdtA antibody showed similar results.
The exact role of CdtA/CdtC heterodimer in CDT toxicity is not yet clearly understood, and further work is required to fully identify the role of CdtA/CdtC heterodimer, especially in its mechanism of host cell binding. Therefore, the soluble CdtA-CdtC protein will serve to investigate the pathological process of A. actinomycetemcomitans and also to be a potential vaccine material. More interestingly, there is a possibility of using CDT as a mucosal adjuvant through its binding activity to cell surface receptor27,28). Up to now, cholera toxin or enterotoxic E. coli toxin has been mainly utilized as a mucosal adjuvant but these toxins belong to AB5 family. But CDT belongs to AB2 and its simple structure and/or lesser toxicity fit to develop a new mucosal adjuvant.
In conclusion, CdtA and CdtC proteins were co-expressed as soluble protein and CdtA-CdtC protein bound to GM1 ganglioside. Further studies will be necessary to characterize precisely CdtA-CdtC protein.
Figures and Tables
Figure 1
SDS-PAGE analysis of purified CdtA and CdtC protein. After expression of recombinant proteins from pRSET-cdtA and pET28a-cdtC, the transformed bacteria were sonicated and centrifuged. The supernatant was loaded onto Ni-NTA agarose column and Flow-through (FT) was saved. The sample-loaded column was washed three times (W1~W3) and eluted (E1~E3) as described in Materials and Methods. rCdtA indicated purified recombinant CdtA protein from pET-cdtA and served as control.
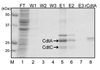
Figure 2
Western blot analysis of purified CdtA and CdtC protein using anti-His6 antibody. rCdtA-CdtC protein was purified from E. coli transformed with both pRSET-cdtA and pET28a-cdtC, while rCdtA from E. coli transformed with pET-cdtA. Protein samples were electrophoresed in SDS-PAGE gel, transferred to nitrocellulose membrane, and detected by anti-His6 antibody.
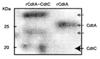
Figure 3
Western blot analysis of purified CdtA and CdtC protein using monoclonal anti-CdtA antibody. Protein samples were purified by Ni-NTA column from bacterial lysates from E. coli transformed with both pRSET-cdtA and pET28a-cdtC (rCdtA-CdtC), and E. coli transformed with pET-cdtA (rCdtA). Samples were separated in SDS-PAGE gel and then transferred to nitrocellulose membrane, and detected by anti-CdtA antibody. Bands around 25 kDa was clearly shown but band at 20 kDa not shown.
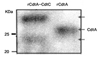
Figure 4
GM1 ELISA of purified CdtA-CdtC protein using anti-His6 antibody. ELISA plate was coated with GM1 ganglioside and further incubated with diverse concentration of CdtA and CdtA-CdtC protein. After washing, the remaining amount of CdtA and CdtA-CdtC protein was quantified using anti-His6 antibody at OD405.

Figure 5
GM1 ELISA of purified CdtA-CdtC protein using monoclonal anti-CdtA antibody. ELISA plate was coated with GM1 ganglioside and further incubated with diverse concentration of CdtA and CdtA-CdtC protein. After washing, the remaining amount of CdtA and CdtA-CdtC protein was quantified using monoclonal anti-CdtA antibody at OD405.

References
1. Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003. 57:29–55.

2. Lara-Tejero M, Galan JE. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 2002. 10:147–152.

3. Leung WK, Ngai VKS, Yau JYY, et al. Characterization of Actinobacillus actinomycetemcomitans isolated from young Chinese aggressive periodontitis patients. J Periodontal Res. 2005. 40:258–268.

4. Ahmed HJ, Svensson LA, Cope LD, et al. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J Med Microbiol. 2001. 50:860–864.

5. Fabris AS, DiRienzo JM, Wikstrom M, Mayer MP. Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitansisolates from geographically diverse populations. Oral Microbiol Immunol. 2002. 17:231–238.

6. Belibasakis G, Johansson A, Wang Y, et al. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur J Oral Sci. 2002. 110:366–373.

7. Kanno F, Korostoff J, Volgina A, DiRienzo JM. Resistance of human periodontal ligament fibroblasts to the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. J Periodontol. 2005. 76:1189–1201.

8. DiRienzo JM, Song M, Wan LSY, Ellen RP. Kinetics of KB and HEp-2 cell responses to an invasive, cytolethal distending toxin producing strain of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002. 17:245–251.

9. Belibasakis GN, Johansson A, Wang Y, et al. The cytolethal distending toxin induces receptor activator of NF-kappaB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect Immun. 2005. 73:342–351.

10. Belibasakis GN, Brage M, Lagergård T, Johansson A. Cytolethal distending toxin upregulates RANKL expression in Jurkat T-cells. APMIS. 2008. 116:499–506.

11. Mogi M, Otogoto J, Ota N, Togari A. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J Dent Res. 2004. 83:166–169.

12. Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994. 62:1046–1051.

13. Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000. 37:952–963.

14. Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004. 429:429–433.

15. Peres SY, Marches O, Daigle F, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997. 24:1095–1107.
16. Lee RB, Hassane DC, Cottle DL, Pickett CL. Interactions of Campylobacter jejuni cytolethal distending toxin subunits CdtA and CdtC with HeLa cells. Infect Immun. 2003. 71:4883–4890.

17. McSweeney LA, Dreyfus LA. Carbohydrate-binding specificity of the Escherichia coli cytolethal distending toxin CdtA-II and CdtC-II subunits. Infect Immun. 2005. 73:2051–2060.

18. Mise K, Akifusa S, Watarai S, et al. Involvement of ganglioside GM3 in G2/M cell cycle arrest of human monocytic cells induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect Immun. 2005. 73:4846–4852.

19. Yamada T, Komoto J, Saiki K, Konishi K, Takusagawa F. Variation of loop sequence alters stability of cytolethal distending toxin (CDT): crystal structure of CDT from Actinobacillus actinomycetemcomitans. Protein Sci. 2006. 15:362–372.

20. Boesze-Battaglia K, Besack D, McKay T, et al. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol. 2006. 8:823–836.

21. Saiki K, Konishi K, Gomi T, Nishihara T, Yoshikawa M. Reconstitution and purification of cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiol Immunol. 2001. 45:497–506.

22. Ko SY, Jeong DK, Ryu SH, Kim HS. Cloning and protein expression of Actinobacillus actinomycetemcomitans cytolethal distending toxin subunit CdtA. J Korean Acad Periodontol. 2007. 37:339–351.

23. Lee ES, Park SY, Lee EU, Kim HS. Cloning and protein expression of Aggregatibacter actinomycetemcomitans cytolethal distending toxin C. J Korean Acad Periodontol. 2008. 38:317–324.

24. Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol. 2002. 4:245–255.

25. Nesic D, Stebbins CE. Mechanisms of assembly and cellular interactions for the bacterial genotoxin CDT. PLoS Pathog. 2005. 1:214–224.

26. Ueno Y, Ohara M, Kawamoto T, et al. Biogenesis of the Actinobacillus actinomycetemcomitans cytolethal distending toxin holotoxin. Infect Immun. 2006. 74:3480–3487.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download