Abstract
Purpose
Synthetic bone products such as biphasic calcium phosphate (BCP) are mixtures of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP). In periodontal therapies and implant treatments, BCP provides to be a good bone reconstructive material since it has a similar chemical composition to biological bone apatites. The purpose of this study was to compare bone regeneration capacity of two commercially available BCP.
Methods
Calvarial defects were prepared in sixteen 9-20 months old New Zealand White male rabbits. BCP with HA and β-TCP (70:30) and BCP with Silicon-substituted hydroxyapatite (Si-HA) and β-TCP (60:40) particles were filled in each defect. Control defects were filled with only blood clots. Animals were sacrificed at 4 and 8 week postoperatively. Histomorphometric analysis was performed.
Results
BCP with HA and β-TCP 8 weeks group and BCP with Si-HA and β-TCP 4 and 8 weeks groups showed statistically significant in crease (P<0.05) in augmented area than control group. Newly formed bone area after 4 and 8 weeks was similar among all the groups. Residual materials were slightly more evident in BCP with HA and β-TCP 8 weeks group.
With the development of implantology, installation of dental implants and rehabilitation in severely atrophic bone area became more routine procedures at dental clinics. However, the need for enough bone support and sufficient bone architecture still exists in most of clinical cases. Among the available materials used for bone reconstruction, autogenous bone is currently the gold-standard because it is a source of osseous matrix, cells, and growth modulating molecules1). However, the use of autogenous bone is limited by donor site morbidity, the quantity of donor bone, frequent infections and unpredictable resorption that may compromise aesthetics and function. To overcome the limits of autogenous bone, various substitutive biomaterials are proposed. Materials of human and animal origin have the potential risk of cross contamination and the disadvantages of limited supply2,3). As a consequence, synthetic products as the biphasic calcium phosphate (BCP) which is a mixture of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) were introduced4-7).
Previous reports have shown that HA particles did not elicit an inflammatory response and they provided a good scaffold for the new bone to grow in. However, HA particles failed to show evidence of new periodontal tissue attachment, osteogenesis, or cementogenesis in the treatment of periodontal osseous defects8-10). It was rather suspected that the material produced a response like a well-tolerated foreign body within the host connective tissue. On the contrary, β-TCP was reported to resorb unpredictably in biologic fields and not to provide a predictable scaffold for new bone to grow in11-13). However the combination of HA and β-TCP and the development of two-phased calcium phosphate or biphasic calcium phosphate ceramic made it possible to control the resorbability of the material and at the same time maintain its osteoconductive property14).
In many cases of bone grafts in implant surgeries, BCP provides good bone reconstruction since it has similar chemical composition to biological bone apatites. Also it has been proved of its efficacy as a bone substitute material in many human clinical applications3,15,16). There has been lots of trials to find ideal combination of HA/β-TCP ratio to maximize bone regeneration capacity. Early studies have shown that approximately 60% of HA and 40% of β-TCP seemed to provide a reasonable bone conductive property3,10,14,17). However the optimal ratio is still not determined. Consequently, many manufactures have come up with their unique combinations of HA/β-TCP.
The Si-substituted macroporous biphasic calcium phosphate (Bonemedik-DM®, META bio med Co. Ltd., Chungwongun, Korea) is a mixture of HA and β-TCP with a ratio of 60 : 40. The material is manufactured by substituting silicon (Si) ions of HA into phosphate (P) sites and mixing β-TCP granules with HA particles to enhance both physical and chemical factors18). The presence of silicon in HA is known to play an important part on the formation of bone19). The overall granule sizes are from 0.25 to 1 mm. The other product on the market (Osteon®, Genoss. Co. Ltd., Suwon, Korea) is a mixture of HA and β-TCP with a ratio of 70 : 30. The granule sizes are from 0.5 to 2 mm.
The purpose of this study was to evaluate bone regeneration capacity of two commercially available biphasic calcium phosphate in rabbit calvarial defect.
Sixteen 9-20 months old New Zealand White male rabbits (Oryctolagus cuniculus) were used. The animals were housed in cages and fed with standard animal food. This study has been reviewed and approved by the Institutional Animals Care and Use Committee, Yonsei Medical Center, Seoul, Korea.
All surgeries were performed under sterile conditions. All rabbits were anesthetized with an intramuscular (IM) injection of a solution of 91% ketamine hydrochloride (Ketalar®, Yuhan Co., Seoul, Korea) and 9% xylazine (Rumpun®, Bayer Korea Ltd., Seoul, Korea). Surgical area of cranium was anesthetized with 2% lidocaine and the scalps were shaved and disinfected. The calvariae were exposed through a mid-line skin incision. The periosteum was retracted laterally and bilaterally, and 8-mm-diameter defects were made in the parietal bones by means of a standardized trephine cutting bur under physiological saline solution irrgation20).
Three defects were made per one animal in triangular shape with an inter defect distance of 3 mm to exclude any influence to different groups. To create the same environments, sagittal suture was avoided when creating defects. The defects were filled with the respective materials, with one cavity destined to the control group containing blood clot. The defect allocation was randomly assigned in each rabbit (Fig. 1). The graft materials were wetted in sterilized saline and gently packed into the defects. Particle size was 1.0~2.0 mm of Osteon® and Bonemedik-DM®.
The periosteum was drawn over the defects and sutured by resorbable suture material (5-0 Vicryl®, Ethicon, Somerville, NJ, USA). Subcutaneous mucosa were adapted and sutured with resorbable suture material (4-0 Vicryl®, Ethicon, Somerville, NJ, USA). Finally the skin was closed with 4-0 absorbable monofilament suture (Monosyn®, Braun, Tuttlingen, Germany). The sutures were removed after 10 days after the confirmation of intact closure of skin. The rabbits were sacrificed after 4 and 8 weeks.
The experimental specimens were obtained by using number 702 bur under physiological saline irrigation. The specimens were fixed in 10% formaldehyde solution for 10 days and they were processed to the routine procedures of slide preparation with 8 µm sections stained with Haematoxylin-Eosin (H-E) to be analyzed under an optical microscope (Olympus BX50, Olympus Optical Co., Tokyo, Japan).
All treatment and control sites healed uneventfully with no clinical evidence of inflammatory response to the graft material. Also the distribution of residual graft materials was homogeneous on the surface of the bone defect, regardless of group (Osteon, Bonemedik-DM).
For the control group, the perforated areas were filled with loose fibrous tissue and a few newly formed bone was observed. A slight decrease in bone in growth towards the center of the defect could be observed compared to test groups (Fig. 2, 3).
For the Osteon group, lamellar bone was apposed in close contact at the surface of the granules and consequent bone ingrowth was observed in both 4 and 8 weeks. However, relatively large particles of Osteon® were evident in 4 weeks which were in the middle of resorption and substitution into new bone. On the contrary, particle size and total area were reduced in 8 weeks. Also the density of newly formed bone was higher compared to 4 week. Fibrous tissues were still observed between the granules (Fig. 4, 5).
For the Bonemedik-DM group, particles were incorporated in mature new bone and were resorbed during the remodeling process in 4 weeks. The individual particles were clearly identifiable and they were surrounded by varying amounts of newly formed bone without being encapsulated by loose fibrous connective tissues. The amount of bone ingrowth was greater than for the Osteon group. Small particles surrounded by new bone were present between the larger ones (Fig. 6, 7).
Relatively irregular inner surface of unresorbed ceramic particles of Bonemedik-DM group indicates that resorption process took place before deposition of mineralized bone matrix. On the contrary, relatively smooth appearing inner surface was found in Osteon group.
Bone substitutes are recognized through bone growth from the host bone to the graft, and progressing from the outer part to the core by osteoconduction. There were osteoblastic and osteoid layers at the border of the defect and around grafted particles in Osteon and Bonemedik-DM groups. The total area of augmentation, bone area, residual material area and soft tissue area were measured.
Total augmented area including bone area, residual materials and soft tissue area was higher in Osteon 4 and 8 week groups and Bonemedik-DM 4 and 8 week groups than the control group (Table 1, Fig. 2, 3). These results were statistically significant (P<0.05). Bone area at 4 week was slightly higher in Bonemedick-DM group which means early bone ingrowth. On the contrary, Osteon group showed more bone area than Bonemedik-DM group. Residual materials were decreased in both Osteon and Bonemedik-DM groups with no statistical significance. Soft tissue area was dominantly wider in Bonemedik-DM groups regardless of weeks. In grafted groups, total amount of bone formation and residual materials exceed or is equal to control groups.
In the scope of bone regeneration, the augmentation of bone defects using autogenous bone to be the gold standard. However, autogenous bone is not always available in sufficient volume according to clinical situations. Therefore various methods such as graft materials and growth factors are proposed to replace autogenous bone. Currently various growth factors such as TGF-β, PDGF, and VEGF are studied to stimulate regeneration of bone tissue in several ways21-23). Also rhBMP-2, recombinant human osteogenic protein-1 (rhOP-1/rhBMP-7), and recombinant human growth/differentiation factor-5 (rhGDF-5) are being pursued as therapies for reconstruction and repair of induced and congenital skeletal defects24,25). However these growth factors are not well documented and commercially unavailable. Therefore, most of clinicians are prone to choose various grafting materials to overcome bone defects in implantology or periodontal therapies in most cases.
Calcium phosphate ceramics have long been investigated as biologically compatible material to used in the treatment of periodontal osseous defects and implant surgery. Especially two-phased calcium phosphate or biphasic calcium phosphate ceramic was developed to control the resorbability of the material and at the same time maintain its osteoconductive property. The in vivo and in vitro dissolution of calcium phosphate ceramics was found to be dependent on the composition, crystallinity, and pH of the solution26). Kwon et al27) reported that biphasic HA/β-TCP composite powders showed average solubility between HA and β-TCP and concluded that the dissolution rate of the calcium phosphate powders was strongly dependent on the β-TCP content. It is well known that early resorption of materials cannot maintain appropriatespace to regenerate bone tissue. On the other hand, the delayed resorption of materials inhibits new bone formation. Therefore, appropriate timing of dissolution of BCP and choosing right ratio of HA/β-TCP is crucial to maximize bone regeneration.
Gauthier et al28) used 60% of HA and 40% of β-TCP in extraction sockets in canine models to report that well formed cortical bone over the materials was present and it inhibited the resorption of alveolar bone after 3 months. Nery et al14) used different ratio of HA/β-TCP in canine models to prove that higher HA ratio showed accelerated new bone formation and new attachment levels.
The results of this study indicated that the bone filling of calvarial defect realized with micro macroporous biphasic calcium phosphate granules after 4 to 8 weeks have moderate bone ingrowth capacity and it appears to demonstrate a pattern that is higher HA ratio to β-TCP tends to show greater bone regeneration as shown histologically. However, statistically significant different was not noted between Osteon and Bonemedik-DM.
Total residual materials were much more abundant in Osteon 8 weeks group. This may be due to the low dissolution rate of porous HA17) and easy resorbability of β-TCP which were proven in vitro29) and in vivo12,30). Therefore the bone quality might be influenced inferiorly by remaining particles. However, these residual materials may stay long enough for cell differentiation, maturation, and revascularization31). Longer period of observation would be required to fully evaluate these results.
The results from this study confirm the resorbability on time of Osteon® and Bonemedik-DM® and the scaffold effect of the HA content and high osteoconduction property. These two crucial properties involved a balance of resorption and bone ingrowth at the expense of the micro-macropours bioceramics.
Although this study showed that the amount of bone regeneration of control sites was higher than graft sites, the BCP grafts also showed reasonable bone ingrowth and can be a good candidate to replace natural bone regeneration and to maintain space for longer period of time. Despite the limitation of this study because of small sample size, we could conclude that the higher portion of HA particles produced longer resorption period and maintained better bone structure. Also, graft of Osteon® and Bonemedik-DM® in the rabbit calvarial defects model was shown to be potentially beneficial at early bone healing.
Figures and Tables
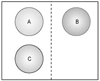 | Figure 1Schematicdrawing of designated material filled in defects. Dotted line represents sagittal suture of cranium; A: Control, B: Bonemedik-DM®, C: Osteon®. |
 | Figure 2Light micrographs of control group at 4 weeks postoperatively. Thick fibrous tissue is covering the defect ×6 (A), ×200 (B). |
 | Figure 3Light micrographs of control group at 8 weeks postoperatively. Mature bone tissue is observed among connective tissue ×16 (A), ×200 (B). |
 | Figure 4Light micrographs of Osteon group at 4 weeks postoperatively. Relatively large particle of residual materials are surrounded obsteoblasts and newly formed bone ×16 (A), ×200 (B). |
 | Figure 5Light micrographs of Osteon group at 8 weeks postoperatively. Relatively small particles are scattered and newly formed bone are surrounding ×16 (A), ×200 (B). |
 | Figure 6Light micrographs of Bonemedik-DM at 4 weeks postoperatively. Active resorption process is observed ×16 (A), ×200 (B). |
 | Figure 7Light micrographs of Bonemedik-DM at 8 weeks postoperatively. Mature bone tissue is apposed interspace of residual materials ×16 (A), ×200 (B). |
References
1. Barboza EP. Clinical and histologic evaluation of the demineralized freeze-dried bone membrane used for ridge augmentation. Int J Periodontics Restorative Dent. 1999. 19:601–607.
2. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.
3. Daculsi G, Passuti N, Martin S, et al. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990. 24:379–396.

4. LeGeros RZ, Parsons JR, Daculsi G, et al. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation-bioresorption. Ann N Y Acad Sci. 1988. 523:268–271.

5. Levin MP, Getter L, Adrian J, Cutright DE. Healing of periodontal defects with ceramic implants. J Clin Periodontol. 1974. 1:197–205.

6. Levin MP, Getter L, Cutright DE, Bhaskar SN. Biodegradable ceramic in periodontal defects. Oral Surg Oral Med Oral Pathol. 1974. 38:344–351.

7. Yukna RA, Cassingham RJ, Caudill RF, et al. Six month evaluation of Calcitite (hydroxyapatite ceramic) in periodontal osseous defects. Int J Periodontics Restorative Dent. 1986. 6:34–45.
8. Froum SJ, Kushner L, Scopp IW, Stahl SS. Human clinical and histologic responses to Durapatite implants in intraosseous lesions. Case reports. J Periodontol. 1982. 53:719–725.

9. Moskow BS, Lubarr A. Histological assessment of human periodontal defect after durapatite ceramic implant. Report of a case. J Periodontol. 1983. 54:455–462.

10. Ellinger RF, Nery EB, Lynch KL. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent. 1986. 6:22–33.
11. Karabuda C, Ozdemir O, Tosun T, Anil A, Olgac V. Histological and clinical evaluation of 3 different grafting materials for sinus lifting procedure based on 8 cases. J Periodontol. 2001. 72:1436–1442.

12. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981. 00:259–278.

13. Jarcho M. Biomaterial aspects of calcium phosphates. Properties and applications. Dent Clin North Am. 1986. 30:25–47.
14. Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992. 63:729–735.

15. Block MS, Kent JN. A comparison of particulate and solid root forms of hydroxylapatite in dog extraction sites. J Oral Maxillofac Surg. 1986. 44:89–93.

16. Lee J, Jung U, Kim C, Choi S, Cho K. Maxillary sinus augmentation using macroporous biphasic calcium phosphate (MBCP™): Three case report with histologic evaluation. J Korean Acad Periodontol. 2006. 36:567–577.

17. Daculsi G, LeGeros RZ, NeryE , Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989. 23:883–894.

18. Bertoni E, Bigi A, Cojazzi G, et al. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J Inorg Biochem. 1998. 72:29–35.

19. Skrtic D, Antonucci JM, Eanes ED, Brunworth RT. Silicaand zirconia-hybridized amorphous calcium phosphate: effect on transformation to hydroxyapatite. J Biomed Mater Res. 2002. 59:597–604.

20. Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990. 1:60–68.

21. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–646.
22. Sandy J, Davies M, Prime S, Farndale R. Signal pathways that transduce growth factor-stimulated mitogenesis in bone cells. Bone. 1998. 23:17–26.

23. Horner A, Bord S, Kemp P, Grainger D, Compston JE. Distribution of platelet-derived growth factor (PDGF) A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone. 1996. 19:353–362.

24. Lee YJ, Jung SW, Chae GJ, Cho KS, Kim CS. The effect of recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system on bone formation in rat calvarial defects. J Korean Acad Periodontol. 2007. 37:397–407.

25. Wikesjo UM, Huang YH, Polimeni G, Qahash M. Bone morphogenetic proteins: a realistic alternative to bone grafting for alveolar reconstruction. Oral Maxillofac Surg Clin North Am. 2007. 19:535–551. vi–vii.

26. Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res. 1983. 17:769–784.

27. Kwon S, Jun Y, Hong S. Synthesis and dissolution behavior of β-TCP and HA/β-TCP composite powders. J Euro Ceram Soc. 2003. 23:1039–1045.

28. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998. 19:133–139.

29. LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988. 2:164–180.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download